Figure 1. eCBs signaling in neurons.
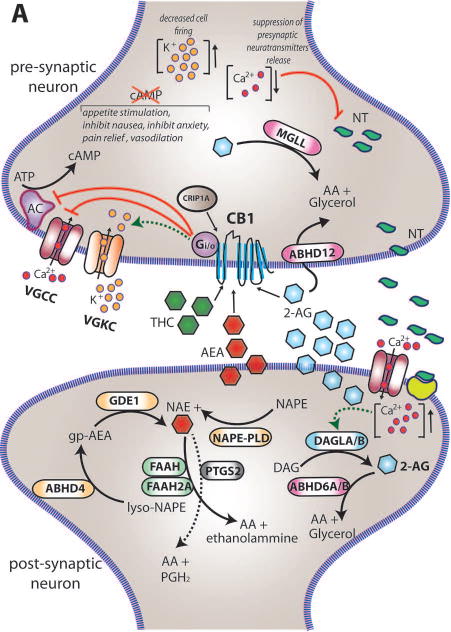
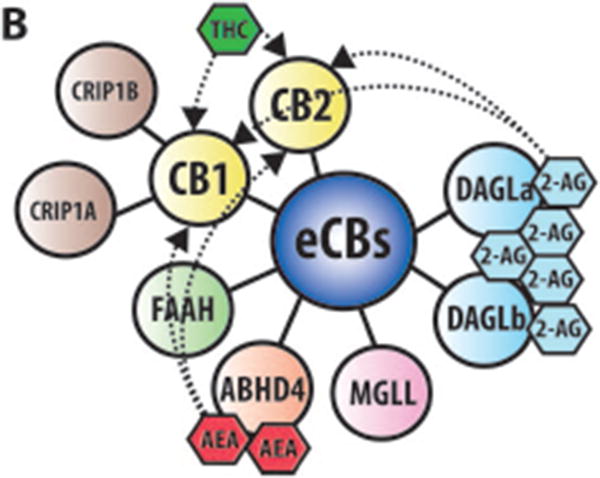
(A) Within the brain the endocannabinoids AEA and 2-AG are biosynthesized from different membrane phospholipid families, both esterified with arachidonic acid (AA). Several possible biosynthetic routes for the formation of AEA in the post-synaptic neuron have been suggested with multiple enzymes implicated: Nacylphosphatidylethanolamine specific phospholipase D (NAPE-PLD), αβ-Hydrolase domain-containing 4 (ABHD4), and glycerophosphodiesterase-1 (GDE1). The biosynthetic precursors of 2-AG are converted to 2-AG by the action of sn-1-diacylglycerol lipases α and β (DAGLα and DAGLβ). Endogenous AEA and 2-AG and exogenous Δ9-THC (THC) activate the CB1 receptor, exposed on the pre-synaptic neuron, causing (1) G-protein mediated inactivation of voltage-gated calcium channels (VGCC) which results in a transient reduction of neurotransmitter (NT) release, (2) G-protein mediated activation of voltage-gated potassium channels (VGKC), which decreases cell firing, and (3) G-protein mediated inhibition of adenylate cyclase (AC) with consequent reduction of cAMP levels. 2-AG is then degraded by three serine hydrolases, MGLL (monoglyceride lipase), ABHD6 (αβ-Hydrolase domain-containing 6) and ABHD12 (αβ-Hydrolase domain-containing 12), that account for approximately 99% of 2-AG hydrolysis in the CNS (Savinainen et al 2012). MGLL is responsible for approx. 85% of 2-AG hydrolysis and co-localizes with CB1 in axon terminals (Savinainen et al 2012). ABHD6 accounts for approx. 4% of brain 2-AG hydrolase activity; in neurons it resides post-synaptically, often juxtaposed with CB1 where it regulates intracellular pools of 2-AG at the site of generation. ABHD12 is highly expressed in microglia and accounts for approx. 9% of total brain 2-AG hydrolysis. AEA is generally degraded by the fatty acid amide hydrolases FAAH and FAAH2A. ProstaglandinEndoperoxide Synthase 2 PTGS2 (COX-2) possesses the capacity to metabolize AEA in vivo and can compete with FAAH for AEA in several brain regions (Glaser & Kaczocha 2010). The cannabinoid receptor interacting protein CRIP1A, transiently interacting with CB1 can stabilize and regulate the inactive state of the receptor (Niehaus et al 2007). In contrast with this conclusion, Guggenhuber and colleagues proposed that CRIP1A regulates CB1 activity in an agonist-dependent manner (Guggenhuber et al 2016). (B) Schematic representation of the main proteins belonging to the eCBs. CB1 and CB2 receptors get activated by endogenous (2-AG and AEA) and exogenous (THC) cannabinoids; 2-AG is synthesised by DAGLα and DAGLβ and degraded by MGLL; AEA is mainly synthesised by ABHD4 and degraded by FAAH. CRIP1A and CRIP1B are known interaction partners of CB1; Levels of 2-AG in the brain are higher than those of AEA.
