Figure 6.
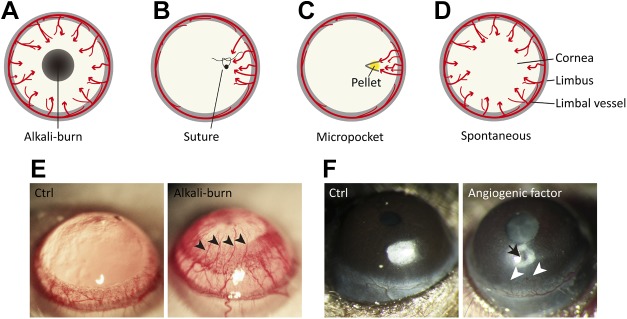
Murine models of corneal angiogenesis. A) Chemical injury model by alkali burn. A filter paper disk that is saturated with 1 N NaOH or control saline solution is applied to the center of the cornea for 30 s, followed by rinsing with saline. At 7–14 d after the alkali burn, corneal angiogenesis can be observed as neovessels growing from the limbus toward the injured region. B) Suture injury model. The cornea is artificially perforated with 10.0 nylon sutures in the experimental animal. Corneal neovascularization can be triggered and observed within 2 wk after the procedure. C) Corneal micropocket assay. A micropocket with a pellet (yellow) that contains proangiogenic factors or vehicle control is created in the stroma of the cornea. At 5–7 d after pellet implantation, corneal neovascularization can be observed and evaluated. D) Transgenic spontaneous models of corneal neovascularization. Genetically engineered mice, such as knockout of Destrin, CD36, or soluble VEGFRs, as well as overexpression of PAX6 in corneas, develop spontaneous corneal neovascularization. E) Representative images of rat corneas that were treated with control solution (Ctrl) or alkali solution-soaked filter papers. The cornea with alkali burn shows neovessels (arrowheads) growing from limbal vessels toward the central corneal burn. F) Mouse corneas with pellet implantation into micropockets. No abnormal corneal angiogenesis is observed with the implant of control buffer–containing pellet, whereas the pellet with angiogenic factor (arrow) induces pathologic vessel growth (arrowheads) from the limbus. Red arrows in panels A–D represent the direction of blood vessel growth.