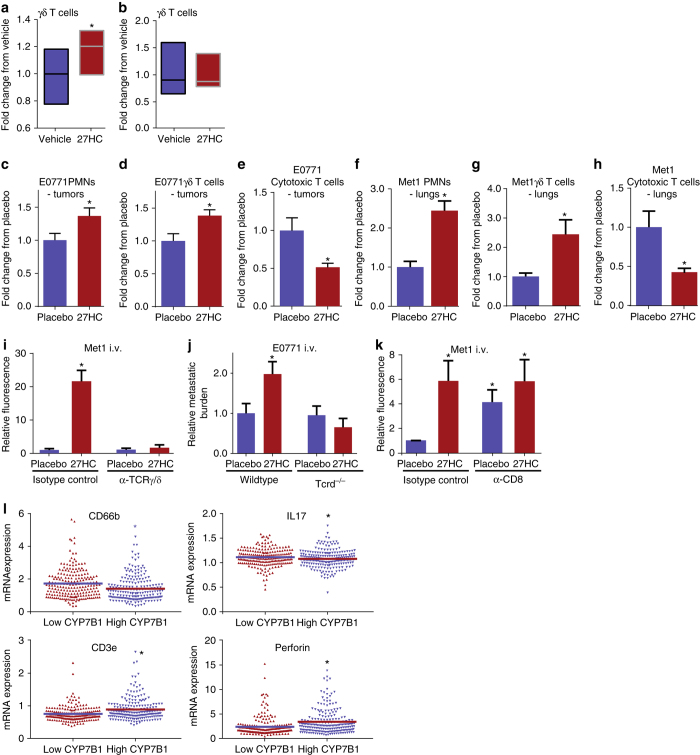
Fig. 4.
27HC increases presence of γδ-T cells and PMNs, and decreases presence of cytotoxic CD8+ T cells within tumors and metastatic lungs. a Bone marrow-derived neutrophils were co-cultured with splenocytes in the presence or absence of 27HC for 4 days. At this time, cells were analyzed by flow cytometry for the presence of γδ-T cells (N = 5/group). b The 27HC stimulated increase in γδ-T cells is not apparent when only splenocytes were cultured. c–e Early-stage E0771 tumors from mice treated with placebo or 27HC were analyzed by flow cytometry for γδ-T cells, PMNs and cytotoxic T cells (placebo N = 10, 27HC N = 9). f–h Early-stage metastatic lungs (Met1) from mice treated with placebo or 27HC were analyzed by flow cytometry (N = 8/group). i γδ-T cells are required for the colonizing effects of 27HC, as immune-depletion of γδ-T cells with an antibody against TCRγδ (α-TCRγ/δ) ablates its effects on Met1 colonization of the lungs (N = 8 except for isotype control 27HC group where N = 10). Mice were pretreated with indicated ligands for 5 days prior to engraftment with Met1 cells expressing iRFP (as in Fig. 2a). j γδ-T cells are required for the colonizing effects of 27HC, as 27HC fails to increase colonization in Tcrd−/− mice (from left to right N = 13, 12, 12, 13). k Immune depletion of CD8+ cells (α-CD8) increases colonization, and exogenous 27HC treatment does not further increase colonization of Met1 cells to the lungs (from left to right: N = 9, 7, 8, 8). l Scatterplot analysis of TCGA data of 421 invasive breast cancer tumors for mRNA expression of CD66b, IL-17, CD3e, and PRF1 (perforin), parsed by median expression of CYP7B1. Results are depicted as mean +/− SEM, with the exception of a, b, and l where the horizontal line depicts the mean. Asterisks denote statistical differences between groups (p < 0.05). [a–h: Unpaired two-tailed student’s t-test. i–k: One-way ANOVA followed by a Student Newman-Keuls multiple comparison test. l: Mann–Whitney test]