Abstract
Purpose
This meta-analysis, following our previous reports those documented an overall 57% diminution in mean sperm concentration around the globe over past 35 years and 32.5% decline in past 50 years in European population, attempts to report the declining trend of sperm concentrations in African population between 1965 and 2015.
Methods
In the course of retrieval of data following MOOSE guidelines and PRISMA checklist, we found a total of fourteen studies that have been conducted during that period on altering sperm concentration in the African male.
Results
Following analysis of the data, a time-dependent decline of sperm concentration (r = −0.597, p = 0.02) and an overall 72.6% decrease in mean sperm concentration was noted in the past 50 years. The major matter of concern is the present mean concentration (20.38×106/ml) is very near to WHO cut-off value of 2010 of 15×106/ml. Several epidemic diseases, genital tract infection, pesticides and heavy metal toxicity, regular consumption of tobacco and alcohol are reported as predominant causative factors.
Conclusion
This comprehensive, evidence-based meta-analysis and systematic review concisely presents the evidence of decreased sperm concentration in the African male over past 50 years with possible causative factors to serve the scientific research zone related to male reproductive health.
Keywords: Semen quality, sperm concentration, sperm count
Introduction
A worldwide decline in sperm count has been presented vastly in past few decades through several studies1,2. The deterioration of semen quality was first noted in 1974 by Nelson and Bunge3. In 1992, Carlsen et al. reported a global decline in sperm counts in a meta-analysis of 61 studies between 1938 and 1990 evaluating the semen analyses of 14,947 presumably fertile men from 23 countries4. In that analysis, they have found significant declines in sperm count in the United States, Europe, and Australia, but no such decline in non-Western countries. Similar declines were also proclaimed by numerous other studies, but were unable to establish a clear cause5,6 But, since then the reports published regarding the changes in human semen parameters were so far inconsistent: Nieschlag et al.7 reported no changes in any parameter7, while Ng et al.8 revealed significantly different seminal volumes in different age groups8. Recently, Rolland et al.9 in their analysis showed 32% decline in sperm count from 1989 to 20059. In our recent articles, we have also reported decline in semen volume10 and sperm count11 in males over past few decades.
Reports regarding the altered sperm concentration in African sub-continent is very limited. The first document regarding altered sperm concentration of the African population after 1965 was put forward by Chukudebelu et al.12. But during 1965–1979, there is no study recounting altering sperm concentration in Africa. But, there are plenty of articles which report causes and risk factors of male infertility in the African population. These reports showed that significantly increased serum follicle stimulating hormone (FSH)13 and decreased inhibin B14 may result in testicular spermatogenic dysfunction15. Changes in sperm count can also occur after occupational and environmental exposure to toxic agents16–19 or from the predisposing factors of the host, such as age20–22.
Thus, the objective of this meta-analysis was to build-up a substantial idea regarding alterations in sperm concentration in the African population by picking the scattered reports of past 50 years, moulding them in sequential pattern, statistically analysing and through the systematic review looking over the linking factors of decreased sperm concentration.
Data extraction and data anaysis
Research articles on humans published in English from 1965 to 2015 were included in this report23–36 (Table 1).
Table 1.
Studies on changes of sperm concentrations in different age groups in past 50 years in Africa.
| Country | Population | Sample size (n) |
Male age definition (range/ mean/ group, in years) |
Direction of effect with increasing age |
Study |
| Nigeria | Cohort study | 53 | 20–45 | ↓ (P<0.01) | Lapido, 1980 |
| Egypt | Andrology lab | 45 | 19–53 | ↓ (P<0.01) | Shaarawy and Mahmoud, 1982 |
| Libya | Infertility clinic | 1500 | 20–45 | ↓ (P<0.01) | Sheriff, 1983 |
| Nigeria | Cohort study | 100 | 20–45 | ↓ (P<0.01) | Osegbe et al., 1986 |
| Libya | Cohort study | 10 | No age data | ↓ (P<0.01) | Sheriff, 1987 |
| Tanzania | Andrology lab | 120 | 19–55 | ↓ (P<0.01) | Kirei, 1987 |
| Nigeria | Cohort study | 20 | 19–53 | ↓(P<0.001) | Sobowale & Akiwumi, 1989 |
| Nigeria | Andrology lab | 21 | 19–24 | ↓ (P<0.05) | Nnatu et al., 1991 |
| Libya | Cohort study | 1250 | 19–53 | ↓ (P<0.01) | Sheriff and Legnain, 1992 |
| Nigeria | Infertility clinic | 170 | 25–40 | ↓ (P<0.001) | Ugwuja et al., 2008 |
| Tunisia | Infertility clinic | 2940 | 20–45 | ↓ (P<0.001) | Feki et al., 2009 |
| Nigeria | Cohort study | 106 | 20–45 | ↓ (P<0.01) | Akande et al., 2011 |
| Nigeria | Infertility clinic | 316 | 20–40 | ↓ (P<0.05) | Jimoh et al., 2012 |
| Tunisia | Andrology lab | 116 | Males of mean age 32.74 |
↔ (NS) | Hadjkacem Loukil et al.,2015 |
Data are represented as Mean(SD); ↓=decrease; ↑=increase; ↔ = no change; NS=not significant at P<0.05, no P value indicates that no statistical testing was done
We also included the reports of Carlsen et al. (1992), i.e. reports from 1965 to 19924. We selected publications about sperm concentration, with pre-defined criteria for inclusion and exclusion, as follows. [1] The non-Carlsen studies published during 1965 to 2015 were identified by using Medical Subject Headings (MeSH) of electronic databases which included Medline, National Library of Medicine, Bathesda, MD with the key words: sperm count, sperm density, sperm concentration, semen quality, male infertility and semen analysis. [2] Relevant literature on changes of the sperm concentration and its influence on future natural and assisted conception cycles were retrieved. [3] Data of the subjects with clinical problems were excluded. [4] Studies with insufficient numbers of subjects (n < 5) were excluded. We followed Meta-analyses of Observational Studies in Epidemiology (MOOSE) guidelines37 and Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) checklist38, 2009 to extract the data using the above mentioned electronic databases (Table 2). In each case sperm concentration and its outcome were evaluated. Analytic epidemiological studies were emphasized. Therefore, the current analysis was based on 14 African studies published in 1965–2015. For simple statistical analyses Microsoft Excel v.2013 was used and correlation and regression analyses of data were done using StatSoft. (2011) and SPSS v.22.0 to calculate correlation coefficient and it was considered to be significant if p was <0.05 or <0.00139. Mean sperm concentrations of all 14 reports were also analyzed with linear regression weighted by number of subjects included in the individual publications.
Table 2.
Flow chart of study selection according to MOOSE guidelines.
State of affairs: past 50 years
African scenario
In 1991, WHO had estimated that almost 20–35 million couples were infertile in Africa40. Nigeria is suggested to have been suffering from highest infertility problems among the other African regions, the male infertility factor accounting for 40–50%. The degree of infertility and its cause vary from place to place. These are evident from the study pursued in mid-Western Nigeria which brought to the lime light that about 50%, of the 780 couples under evaluation, differed in the causes of their infertility41. Study associated with South-Western Nigeria reported that 42.4% infertility resulted from the male factor42.
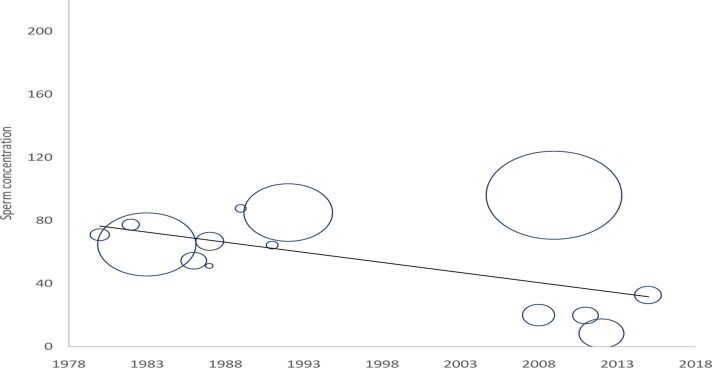
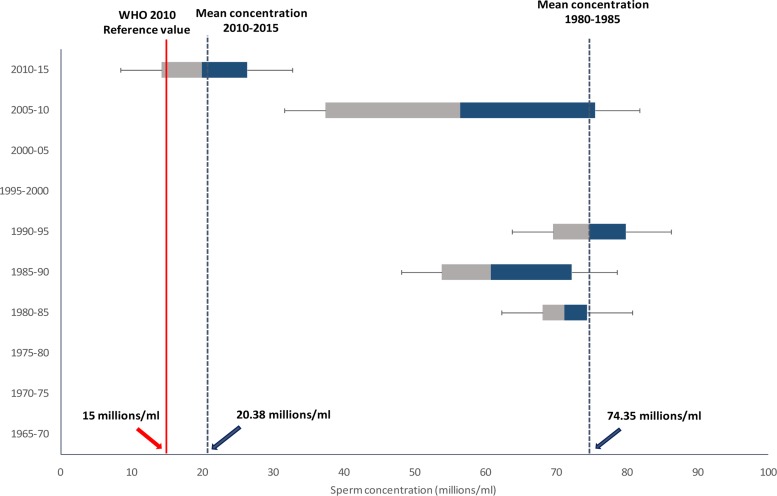
During the retrieval of relevant documents, we found only fourteen studies which had been conducted on alterations of sperm concentration of African population in the last 50 years. The outcome of these studies is represented in Table 1. Most of the reports were based on epidemiological studies (43%), and others included andrology laboratories (29%) and infertility clinics (29%). Among the 14 published research works discussed in this article from 1965 to 2015, most were carried out in Nigeria. Most of the studies used sample size less than 500 men (79%) and only three studies included sample size >1000 (21.43%). Out of 14 reports, 93% have provided data about the age of subjects; all of these reports depicted significant decrease in sperm concentration from 1965 to 2015 while only one report showed no significant alteration. We also recorded that most of the studies were carried out in Nigeria. A time-dependent decline in sperm concentration was observed from 1965 to 2015 (r = −0.597, p = 0.02; Fig 1) that reflected an overall 73% decrease in sperm concentration (Fig 2).
Fig 1.
Temporal decline in sperm concentration (×106/ml), bubble size corresponds to the number of men in study following Table 1
Fig 2.
Box and whisker plot of sperm concentration data of African men between 1965 and 2015 with WHO cut-off value (2010).
It is thus understandable that regional variations in reproductive status prevails in Africa and the high rates of male infertility in Nigeria is thought to be due to infections, sexually transmitted diseases and hormonal abnormalities43,44. But, the major matter of concern is that the present mean concentration (20.38×106/ml) is very near to WHO cut-off value of 2010 of 15×106/ml.40
Comparing with global scenario
In one of our recently published article, we showed a significant decrease in sperm concentration worldwide between 1980 and 2015 from 91.65×106/ml to 39.34×106/ml (r = −0.313, p = 0.0002). It reflected almost 57% decline in sperm count worldwide from 1980. It also showed that recruitment of larger population for this type of study increased predominantly after 199511. In another report, we revealed a time-dependent decline of sperm concentration (r = −0.307, p = 0.02) in European men from 1965 to 2015 and an overall 32.5% decrease in mean concentration45.
Recent studies on male reproductive system when brought together bring conflicting evidence to the forefront regarding sperm counts with some showing significant decline while some found no change. North America, Europe and Asia were more prone to a declining trend of sperm counts over the years whereas studies based on South America and Australia do not depict such a trend11. However, in this present analysis a significant decline in sperm concentration has been noted in the African population. It has been suggested that these regional differences in sperm counts possibly are biologically meaningful. Most of the controversies that aroused from the past clinical studies about semen quality may be partly due to involvement of only few selected groups of men. In many studies, historical data collected for other purposes has been used without close attention to important and specific factors relevant to an analysis of secular or geographical trends.
Possible causative factors
Although little is known about the causative factors for the decline in sperm count worldwide, significant associations have been reported between impaired semen quality including sperm count, and several of the etiological factors in developing countries especially Africa46. We also reported some link factors of declining sperm concentration worldwide11, but the proper correlation with a single factor is difficult to establish.
In Africa, it has been reported that some epidemic diseases, like malaria, Schistosomiasis and viral infections play pivotal roles in the declining sperm concentration47,48. Yeboah et al. in 1992 reported that in Ghana they found a 12% higher incidence of inflammatory testicular or prostatic conditions as compared with those found in Europeans, suggesting that inflammatory conditions contribute more to male infertility and declining sperm concentrations in Africa.47 Genital tract infections and sexually transmitted infections are associated with declining sperm concentrations in African men49,50. Okonofua et al. found that infertile and sub-fertile men are more likely than fertile men to report having experienced penile discharge, painful micturition and genital ulcers, yet they are less likely to present to a formal health institution to seek treatment.50 Therefore, poor health-seeking behaviour of our African men is key to the declining sperm concentration41,50.
Regular alcohol consumption and tobacco smoking are responsible for the declining sperm concentration50. Several studies reported that these two factors are the principal causes of hypogonadism and could cause testicular failure51. Therefore, it is possible that the increased consumption of alcohol and tobacco smoking could be a contributory factor to the global fertility crisis in the human species52. Moreover, tobacco smoking increases intake of cadmium, because the tobacco plant absorbs the metal. Cadmium, being chemically similar to zinc, may replace zinc in the DNA polymerase, which plays a critical role in sperm production53. Geographic differences in the amount of naturally occurring cadmium have been correlated with incidence rates of prostate cancer54. Major changes in the levels of toxic elements in seminal fluid have been related to abnormal spermatozoa function and fertilizing capacity55. Cadmium has beendetected in significantly high levels in serum of men who were smokers and thus implicated this metal as one of the causes of asthenoteratozoospermia55.
Cigarette smoking is an important variable when considering the effect of both lead and cadmium exposure on human health. A single cigarette has been reported to contain 1.5µg of cadmium. Moreover, one tenth of the metal content of a cigarette is inhaled56. Unlike in most developed nations, there are no smoking restrictions in Africa. Even where there are, they are not obeyed. Cumulative evidence suggests that cigarette smoking has a deleterious effect on male fertility by reducing sperm production, motility, and increasing the number of abnormal sperm57. Smokers are 60% more likely to be infertile compared to non-smokers. Cigarette smokers were also shown to have higher levels of circulating estradiol and decreased levels of LH, FSH, and prolactin compared to non-smokers, all of these negatively impact spermatogenesis57.
Emokpae et al. in Kano, Nigeria reported that endocrine abnormalities are common in the infertile males58. Hormonal abnormalities were detected in 22% of oligospermic, 40.7% of severe oligospermic, and 43 of azoospermic subjects. Similarly, hormonal imbalance was also found by Ozoemena et al. in Enugu to be significantly associated with declining sperm concentration and male infertility59. They demonstrated that as much as 80.1% of their subjects were found to have a hormonal imbalance and recommended that hormonal profile should be considered as the gold standard for diagnosis and management of male infertility. An observational retrospective study conducted on 1,201 men (mean age of 35.7 years) in Northern Nigeria investigated for infertility at University of Maiduguri Teaching Hospital, over a two-year period, (2004–2006) showed that 96 (7.9%), underwent hormonal assessment because of abnormalities of their sperm counts. 68 (71%) patients had primary infertility and 72 (75%) had azoospermia. 88 (92%) patients had abnormal hormonal assays, giving a prevalence of endocrine abnormality of 7.3%60.
Therefore, endocrinopathy is also common among infertile Nigerian men as with their counterparts elsewhere. However, the prevalence of endocrinopathy of 7.3% was lower than that reported from Kenya61, an African country, but higher than that reported in Brazil a developing country like Nigeria62.
Numerous anti-oxidant nutrients such as vitamin C, vitamin E, glutathione and co-enzyme Q10 have been documented in several studies as having modulatory effects on sperm parameters52,63. These positive effects may not be observed in Africa because of the well-recognised deficiency of protective micronutrients in this region64. Studies have shown that the concentration of ascorbic acid in seminal plasma directly reflects dietary intake, and lower levels of vitamin C may lead to infertility and increased damage to the sperm genetic material65. Ebesunun et al. determined ascorbate levels in the plasma of 27 Nigerian males with inadequate spermatogenesis66. There were significant decreases in the seminal and plasma ascorbic acid concentration in males who had inadequate spermatogenesis compared with the control values and the author concluded that semen ascorbate levels may play a significant role in reduced sperm characteristics in these patients52,66. Selenium and glutathione are essential to the formation of phospholipid glutathione peroxidase, an enzyme present in spermatids, which becomes a structural protein comprising over 50% of the mitochondrial capsule in the midpiece of mature spermatozoa. Deficiencies of either substance can lead to instability of the midpiece, resulting in defective motility52,63. Akinloye et al. in their study observed a significant inverse correlation between serum selenium level and sperm count. Similarly, seminal plasma selenium correlated with spermatozoa motility, viability, and morphology67.
The spermatotoxic effects of dibromochloropropane (DBCP), a nematocide widely used in agriculture was reported in the early 1960s in rodents by animal toxicologist but their report went essentially unnoticed until the late 1970s when oligospermia and azoospermia were reported in manufacturing plant workers and pesticides applicators68. It was noted that there was limited childbearing among the workers after they started working in DBCP production. About half of the DBCP-exposed azoospermic men remained that way for many years suggesting that all of the stem spermatogonia may have been compromised. 71 others experienced a recovery in their sperm count, but in some cases the recovery did not occur until 3 to 6 years later68. Furthermore, the men had high levels of FSH and LH in serum indicating that DBCP action is directly on the Leydig cells causing alterations in androgen production and action68. Other pesticides such as dichloro-diphenyl-trichloroethane (DDT), endosulphan, and organophosphorus pesticides i.e. malathion, have been reported to show male-mediated adverse reproductive outcomes such as abortions, stillbirths, congenital defects etc. among occupationally exposed workers69.
A significantly higher level of asthenozoospermia and teratozoospermia was found in 2, 4-dichlorophenoxy acetic acid exposed workers as compared to unexposed control subjects70. Although DDT production has been banned in the United States for more than 2 decades, new factories are still being built to produce in some developing nation. The presence of these chemicals in some developing countries is of concern since they are probably accumulating to harmful levels. Ibeh et al. reported higher concentrations of aflatoxin B1 (AFB1) in the semen of infertile Nigerian men than those levels in fertile controls and concluded that the consumption of AFB1 contaminated diets may predispose to male infertility in Nigeria71. Over five billion people in developing countries worldwide are at risk of chronic exposure to AFB1 through food products contaminated by the fungal moulds. The infertile men with aflatoxin in their semen showed a higher percentage of spermatozoa abnormalities (50%) than the fertile men (10–15%). The above observations therefore suggest that pesticides, industrial chemicals and mycotoxins like aflatoxins might be implicated in the declining fertility of the African men71.
A number of occupations are being reported as risk factors for male infertility. For example, an insult to spermatogenesis has been reported among professional drivers who are exposed to the products of fuel combustion, noise, vibration, emotional stress, physical load on the pelvic organs and increased temperature in the pelvis because of prolonged sitting72. Intense exposure to heat in the workplace e.g. working in furnaces or in bakeries, long soaks in the bath tub, use of laptops, and excessive bicycling can cause the temperature in the scrotum to increase enough to impair sperm production. Welders are also at risk due to their exposure to heat, solvents, heavy metals and noise72. Men who wear tight pants which hold the testes close to the body also vulnerable to these defects73. Noticeable improvement in sperm count has been observed when the tight underwear is discarded.
Conclusion
The current meta-analysis, with pertinent evidence reports an overall 73% decline in sperm concentration in African men over past 50 years and the current concentration is very near to WHO cut-off value of 2010 which is a major issue of concern. It also explains the major possible causes of the declining trend. Poorly treated sexually transmitted infections (STIs) and hormonal abnormalities, consumption of excessive alcohol and tobacco smoking are reported as the major causes. But, according to the published articles describing the link factors of male infertility in Africa, exposure to pesticides and heavy metals are the principal triggers of decreased sperm concentration. However, as more than one factor is involved in this decreasing trend, correlation with a single factor is difficult to establish. Conceivably in future with the development of more sensitive biomarkers, we will be able to relate these factors with decreasing sperm concentration precisely.
Acknowledgements
The authors are thankful to Datuk, Dr. Abdul Gani Bin Mohammed Din, Dean, Faculty of Medicine and Deputy Vice Chancellor (Academic), Lincoln University College and Prof. Dr. Amiya Bhaumik, CEO and Vice Chancellor, Lincoln University College for their kind support and encouragement. Authors are also thankful to Dr. Sandeep Poddar, Research Manager, Lincoln University College for his valuable suggestions during this research work.
Conflict of interest
None
Funding source
None
References
- 1.Sengupta P. Current trends of male reproductive health disorders and the changing semen quality. Int J Prev Med. 2014;5:1–5. PubMed. [PMC free article] [PubMed] [Google Scholar]
- 2.Sengupta P. Recent trends in male reproductive health problems. Asian J Pharm Clin Res. 2014;7:1–5. [Google Scholar]
- 3.Nelson CMK, Bunge RG. Semen analysis-evidence of changing parameters of male fertility potential. Fertil Steril. 1974;25:503–507. doi: 10.1016/s0015-0282(16)40454-1. PubMed. [DOI] [PubMed] [Google Scholar]
- 4.Carlsen E, Giwercman A, Keiding N, Skakkebaek NE. Evidence for decreasing quality of semen during past 50 years. Brit Med J. 1992;305:609–613. doi: 10.1136/bmj.305.6854.609. PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Swan SH, Elkin EP, Fenster L. Have sperm densities declined? A reanalysis of global trend data. Environ Health Perspect. 1997;105:1228–1232. doi: 10.1289/ehp.971051228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Auger J, Kunstmann JM, Czyglik F, Jouannet P. Decline in semen quality among fertile men in Paris during the past 20 years. New Eng J Med. 1995;332:281–285. doi: 10.1056/NEJM199502023320501. PubMed. [DOI] [PubMed] [Google Scholar]
- 7.Nieschlag E, Lammers U, Freischem C, Langer K, Wickings E. Reproductive functions in young fathers and grandfathers. J Clin Endocrinol Metab. 1982;55:676–681. doi: 10.1210/jcem-55-4-676. [DOI] [PubMed] [Google Scholar]
- 8.Ng KK, Donat R, Chan L, Lalak A, Pierro ID, Handelsman DJ. Sperm output of older men. Hum Reprod. 2014;19:1811–1815. doi: 10.1093/humrep/deh315. [DOI] [PubMed] [Google Scholar]
- 9.Rolland M, Le Moal J, Wagner V, Royère D, De Mouzon J. Decline in semen concentration and morphology in a sample of 26609 men close to general population between 1989 and 2005 in France. Hum Reprod. 2013;28:462–470. doi: 10.1093/humrep/des415. PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sengupta P. Reviewing reports of semen volume and male ageing in last 33 years: from 1980 through 2013. Asian Pac J Repro. 2015;4:242–246. PubMed. [Google Scholar]
- 11.Sengupta P, Dutta S, Krajewska-Kulak E. The disappearing sperms: analysing the reports published between 1980 and 2015. Am J Men's Health. 2016 doi: 10.1177/1557988316643383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chukudebelu WO, Esege N, Megafu U. Etiological factors in infertility in Enugu, Nigeria. Infertility. 1979;2:193–200. [PubMed] [Google Scholar]
- 13.Luetjens CM, Rolf C, Gassner P, Werny JE, Nieschlag E. Sperm aneuploidy rates in younger and older men. Hum Reprod. 2002;17:1826–1832. doi: 10.1093/humrep/17.7.1826. PubMed. [DOI] [PubMed] [Google Scholar]
- 14.Mahmoud AM, Goemaere S, El-Garem Y, Van Pottelbergh I, Comhaire FH, Kaufman JM. Testicular volume in relation to hormonal indices of gonadal function in community-dwelling elderly men. J Clin Endocrinol Metab. 2003;88:179–184. doi: 10.1210/jc.2002-020408. [DOI] [PubMed] [Google Scholar]
- 15.Sengupta P, Banerjee R. Environmental toxins: alarming impacts of pesticides on male fertility. Hum Exp Toxicol. 2014;33:1017–1039. doi: 10.1177/0960327113515504. PubMed. [DOI] [PubMed] [Google Scholar]
- 16.Dutta S, Joshi KR, Sengupta P, Bhattacharya K. Unilateral and bilateral cryptorchidism and its effect on the testicular morphology, histology, accessory sex organs and sperm count in Laboratory Mice. J Hum Repro Sci. 2013;6:106–110. doi: 10.4103/0974-1208.117172. PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kidd SA, Eskenazi B, Wyrobek AJ. Effects of male age on semen quality and fertility: A review of the literature. Fertil Steril. 2001;75:237–248. doi: 10.1016/s0015-0282(00)01679-4. PubMed. [DOI] [PubMed] [Google Scholar]
- 18.Bhattarai T, Chaudhuri P, Bhattacharya K, Sengupta P. Effect of progesterone supplementation on post-coital unilaterally ovariectomized superovulated mice in relation to implantation and pregnancy. Asian J Pharm Clin Res. 2014;7(1):29–31. [Google Scholar]
- 19.Chandra AK, Goswami H, Sengupta P. Dietary calcium induced cytological and biochemical changes in thyroid. Env Toxicol Pharmacol. 2012;34(2):454–465. doi: 10.1016/j.etap.2012.06.003. [DOI] [PubMed] [Google Scholar]
- 20.Spandorfer SD, Avrech OM, Colombero LT, Palermo GD, Rosenwaks Z. Effect of parental age on fertilization and pregnancy characteristics in couples treated by intracytoplasmic sperm injection. Hum Reprod. 1998;13:334–338. doi: 10.1093/humrep/13.2.334. PubMed. [DOI] [PubMed] [Google Scholar]
- 21.Wyrobek AJ, Gordon LA, Burkhart JG, Francis MW, Kapp RW, Letz G, Malling HV, Topham JC, Whorton MD. An evaluation of human sperm as indicators of chemically induced alterations of spermatogenic function. A report of the U.S. Environmental Protection Agency Gene-Tox Program. Mutat Res. 1983;115:73–148. doi: 10.1016/0165-1110(83)90015-5. PubMed. [DOI] [PubMed] [Google Scholar]
- 22.Krajewska-Kulak E, Sengupta P. Thyroid function in male infertility. Front Endocrinol. 2013;4:1–2. doi: 10.3389/fendo.2013.00174. PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lapido OA. [Seminal analysis in fertile and infertile Nigerian men.] Natl Aled Assoc. 1980;72:785–789. PubMed. [PMC free article] [PubMed] [Google Scholar]
- 24.Shaarawy M, Mahmoud KZ. Endocrine profile and semen characteristics in male smokers. Fertil Steril. 1982;38:255–257. doi: 10.1016/s0015-0282(16)46470-8. PubMed. [DOI] [PubMed] [Google Scholar]
- 25.Sheriff DS. Setting standards of male fertility. I. Semen analyses in 1500 patients-a report. Andrologia. 1983;15:687–692. doi: 10.1111/j.1439-0272.1983.tb00194.x. PubMed. [DOI] [PubMed] [Google Scholar]
- 26.Osegbe DN, Amaku EO, Nnatu SN. Are changing semen parameters a universal phenomenon? Eur Urol. 1986;12:164–168. doi: 10.1159/000472607. PubMed. [DOI] [PubMed] [Google Scholar]
- 27.Sheriff DS. Semen analyses in Hansen's disease. Trans R Soc Trop Med Hyg. 1987;81:113–114. doi: 10.1016/0035-9203(87)90299-9. [DOI] [PubMed] [Google Scholar]
- 28.Kirei BR. Semen characteristics in 120 fertile Tanzanian men. East Afr Med J. 1987;64:453–457. PubMed. [PubMed] [Google Scholar]
- 29.Sobowale OB, Akiwumi O. Testicular voltnie and seminal fluid profile in fertile and infertile males in Ilorin, Nigeria. IntJ Gynecol Obstet. 1989;28:155–161. doi: 10.1016/0020-7292(89)90476-1. PubMed. [DOI] [PubMed] [Google Scholar]
- 30.Nnatu SN, Giwa-Osagie OF, Essien EE. Effect of repeated semen ejaculation on sperm quality. Clin Exp Obstet Gynecol. 1991;18:39–42. [PubMed] [Google Scholar]
- 31.Sheriff DS, Legnain M. Evaluation of semen quality in a local Libyan population. Indian J Physiol Pharmacol. 1992;36:83–87. [PubMed] [Google Scholar]
- 32.Ugwuja EI, Ugwu NC, Ejikeme BN. Prevalence of low sperm count and abnormal semen parameters in male partners of women consulting at infertility clinic in Abakaliki, Nigeria. Afr J Reprod Health. 2008;12:67–73. PubMed. [PubMed] [Google Scholar]
- 33.Feki NC, Abid N, Rebai A, Sellami A, Ayed BB, Guermazi M, Bahloul A, Rebai T, Ammar LK. Semen quality decline among men in infertile relationships: experience over 12 years in the South of Tunisia. J Androl. 2009;30:541–547. doi: 10.2164/jandrol.108.005959. PubMed. [DOI] [PubMed] [Google Scholar]
- 34.Akande T, Isah HS, Sekoni VO, Pam IC. The Semen of Fertile Men in Jos, Nigeria. J Med Lab Sci. 2011;20:33–36. PubMed. [Google Scholar]
- 35.Jimoh AAG, Olawui TS, Olaiya Omotoso GO. Semen parameters and hormone profile of Men investigated for Infertility at Midland Fertility Centre, Ilorin, Nigeria. J Basic Appl Sci. 2012;8:16–19. PubMed. [Google Scholar]
- 36.Hadjkacem Loukil L, Hadjkacem H, Bahloul A, Ayadi H. Relation between male obesity and male infertility in a Tunisian population. Andrologia. 2015;47:282–285. doi: 10.1111/and.12257. [DOI] [PubMed] [Google Scholar]
- 37.Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. Meta-analyes of observational studies in epidemiology: a proposal for reporting. JAMA. 2000;283:2008–2012. doi: 10.1001/jama.283.15.2008. PubMed. [DOI] [PubMed] [Google Scholar]
- 38.Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009;6(7):e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fisher RA, Yates R. Statistical Tables for Biological, Agricultural and Medical Research. London: Longman Group; 1974. [Google Scholar]
- 40.World Health Organization, author. Infertility: A tabulation of available data on prevalence of primary and secondary infertility. Programme on material and Child Health and Family Planning Division of Family Health. Geneva: World Health Organization; 1991. [Google Scholar]
- 41.Okonofua F, Menakaya U, Onemu SO, Omo-Aghaja LO, Bergstrom S. A Case-control study of risk factors for male infertility in Nigeria. Asian J Androl. 2005;7:351–361. doi: 10.1111/j.1745-7262.2005.00046.x. PubMed. [DOI] [PubMed] [Google Scholar]
- 42.Ikechebula JI, Adinma JI, Orie EF, Ikegwuonu SO. High prevalence of male infertility in South Eastern Nigeria. J Obstet Gynaecol. 2003;23:657–659. doi: 10.1080/01443610310001604475. PubMed. [DOI] [PubMed] [Google Scholar]
- 43.Akinloye O, Grommok J, Nieschlag E, Simoni M. Androgen receptor gene CAG and GGN polymorphysms in infertile Nigerian men. J Endocrinol Invest. 2009;32:797–804. doi: 10.1007/BF03345748. PubMed. [DOI] [PubMed] [Google Scholar]
- 44.Emokpae MA, Uadia PO, Omale-Itodo A, Orok TN. Male infertility and endocrinopathies in Kano, Northern Nigeria. Ann Afr Med. 2007;6:61–67. doi: 10.4103/1596-3519.55714. PubMed. [DOI] [PubMed] [Google Scholar]
- 45.Sengupta P, Borges E, Jr, Dutta S, Krajewska-Kulak E. Decline in sperm count in European men during the past 50 years. Hum Exp Toxicol. 2017 doi: 10.1177/09603327117703690. [DOI] [PubMed] [Google Scholar]
- 46.Omo-Aghoja L. Male Factor Infertility in Infertility and Assisted Conception in the Tropics. 1st Edition. Delta Reproductive Health Initiative and Research Center; 2015. pp. 39–50. [Google Scholar]
- 47.Yeboah ED, et al. Etiological factors of male infertility in Africa. Int J Fertil. 1992;37(5):300–307. [PubMed] [Google Scholar]
- 48.Masarani M, Wazait H, Dinneen M. Mumps Orchitis. J Royal Soc Med. 2006;99(11):573–575. doi: 10.1258/jrsm.99.11.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Inhorn MC, Buss KA. Ethnography, Epidermiology and Infertility in Egypt. Social Sci Med. 1994;39(5):671–686. doi: 10.1016/0277-9536(94)90023-x. [DOI] [PubMed] [Google Scholar]
- 50.Okonofua F, Omo-Aghoja LO, Menakaya U, Onemu SO, Bergstrom S. A Case-Control Study of Risk Factors for Male Infertility in Southern Nigeria. Trop J Obstet Gynaecol. 2005;22:136–143. doi: 10.1111/j.1745-7262.2005.00046.x. [DOI] [PubMed] [Google Scholar]
- 51.Emanuelle MA, Emanuelle NV. Alcohol and the male reproductive system. Alcohol Res Health. 2001;25:282–287. PubMed. [PMC free article] [PubMed] [Google Scholar]
- 52.Abarikwu SO. Causes and Risk Factors for Male-Factor Infertility in Nigeria: A Review. Afr J Reprod Health. 2013;17(4):150–166. [PubMed] [Google Scholar]
- 53.Emsley J. Nature's building blocks: An A-Z guide to the elements. Oxford [Oxfordshire]: Oxford University Press; 2001. p. 76. [Google Scholar]
- 54.Angwafo PF. Migration and prostate cancer: an international perspective. J Nat Med Assoc. 1998;90:S720–S723. [PMC free article] [PubMed] [Google Scholar]
- 55.Omu AE, Dashtu H, Mohammed AT, et al. Significance of trace elements in seminal fluid of infertile men. Nutrition. 1995;11:502–505. PubMed. [PubMed] [Google Scholar]
- 56.Sram BI, Binkova B, Dejmek J, et al. Teplice program-the impact of air pollution in human health. Env Health Persp. 1996;104:699–714. doi: 10.1289/ehp.104-1469669. PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Olayemi FO. A review on some causes of male infertility. Afr J Biotechnol. 2010;20:2834–2842. PubMed. [Google Scholar]
- 58.Emopkae MA, Uadia PO, Omale-Itodo A, Orok TN. Male Infertility and Endocrinopathies in Kano, Northwestern Nigeria. Ann Afr Med. 2007;6:64–67. doi: 10.4103/1596-3519.55714. PubMed. [DOI] [PubMed] [Google Scholar]
- 59.Ozoemena OFN, Ezugworie JO, Mbah AU, Ejezie FE. Abnormalities of Pituitary gonadal axis among Nigerian males with infertility: Study of patterns and possible etiologic interrelationships. J Urol. 2011;3:133–137. doi: 10.2147/OAJU.S22916. PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Geidam AD, Yawe KDT, Adebayo AEA, et al. Hormonal Profile of men investigated for infertility at the University of Maiduguri in Northern Nigeria. Singapore Med J. 2008;49:538–541. PubMed. [PubMed] [Google Scholar]
- 61.Muthuuri JM. Male infertility in a private Kenyan hospital. East African Medical Journal. 2005;82:362–366. [PubMed] [Google Scholar]
- 62.Pasqualotto FF, Pasqualotto EB, Sobreiro BP, et al. Clinical diagnosis in men undergoing infertility investigation in a University hospital. Urol Int. 2006;76:122–125. doi: 10.1159/000090873. PubMed. [DOI] [PubMed] [Google Scholar]
- 63.Sinclair S. Male Infertility: Nutritional and environmental consideration. Alt Med Rev. 2000;5:28–38. PubMed. [PubMed] [Google Scholar]
- 64.Underwood BA, Smitasiri S. Micronutrients malnutrition: policies and programs for control and their implications. Ann Rev Nutr. 1999;19:303–324. doi: 10.1146/annurev.nutr.19.1.303. PubMed. [DOI] [PubMed] [Google Scholar]
- 65.Dabrowski K, Ciereszko A. Ascorbic acid protects against male infertility in a teleost fish. Experientia. 1996;52:97–100. doi: 10.1007/BF01923351. PubMed. [DOI] [PubMed] [Google Scholar]
- 66.Ebunusun MO, Solademi BA, Shittu OB, et al. Plasma and semen ascorbic levels in spermatogenesis. West Afr J Med. 2004;23:290–293. doi: 10.4314/wajm.v23i4.28143. PubMed. [DOI] [PubMed] [Google Scholar]
- 67.Akinloye O, Arowojolu AO, Shittu OB, et al. Selenium status of idiopathic infertile Nigerian males. Biol Trace Elem Res. 2005;104:9–18. doi: 10.1385/BTER:104:1:009. PubMed. [DOI] [PubMed] [Google Scholar]
- 68.Slutsky M, Levin JL, Levy BS. Azoospermia and Oligospermia among a large cohort of DBCP applicators in 12 countries. Int J Occup Env Health. 1999;5:116–122. doi: 10.1179/oeh.1999.5.2.116. [DOI] [PubMed] [Google Scholar]
- 69.Rupa DS, Reddy PP, Reddy OS. Reproductive performance in population exposed to pesticides in cotton fields in India. Environ Res. 1991;55:123–128. doi: 10.1016/s0013-9351(05)80168-9. PubMed. [DOI] [PubMed] [Google Scholar]
- 70.Lerda D, Rizzi R. Study of Reproduction function in persons occupationally exposed to 2, 4-dichlorophenoxyacetic acid (2, 4-D) Mutat Res. 1991;262:47–50. doi: 10.1016/0165-7992(91)90105-d. PubMed. [DOI] [PubMed] [Google Scholar]
- 71.Ibeh IN, Uraih N, Ogonar JI. Dietary exposure to aflatoxin in human male infertility in Benin City, Nigeria. Int J Fert. 1994;39:208–214. PubMed. [PubMed] [Google Scholar]
- 72.Sheiner EK, Sheiner E, Hammel RD, et al. Effect of occupational exposures on male fertility: Literature Review. Indus Health. 2003;41:55–62. doi: 10.2486/indhealth.41.55. PubMed. [DOI] [PubMed] [Google Scholar]
- 73.Foster WJ, Rowley MJ. Testicular biopsy in the study of gorilla infertility. Am J Primatol. 2005;3:121–125. PubMed. [Google Scholar]