Table 2. Catalytic activity of switchable catalysts 1 and 2 compared to previously reported data for related but non-switchable organocatalysts. Conditions: nucleophile (1 equiv.), electrophile (10 equiv.), catalyst (5 mol%), CDCl3, r.t., 24 h, except entries 2 (50 °C, 48 h, d8-toluene), 4 (1 week, catalyst (25 mol%)) and 6 (CD2Cl2). The product of entry 2 was isolated as a single diastereomer (determined by 1H NMR and HPLC) with catalyst 1 producing (S,R,R) as the major enantiomer. Enantiomers assigned by reference to literature.
| Entry | Reagents | Product a | Conv. (S : R), 1 | Conv. (S : R), 2 | Prev. report b (S : R) |
| 1 |
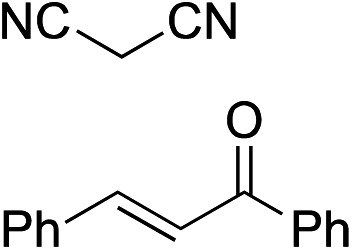
|
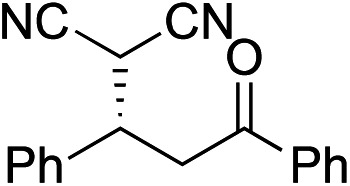
|
91% (83 : 17) | 90% (23 : 77) | 82% (95 : 5)10c |
| 2 |
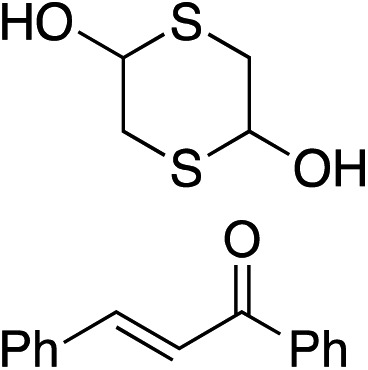
|
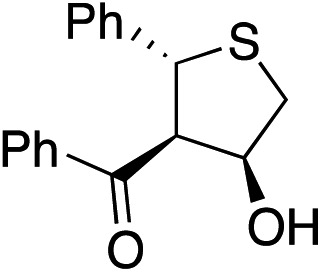
|
93% (25 : 75) | 94% (70 : 30) | 81% (94 : 6)10d |
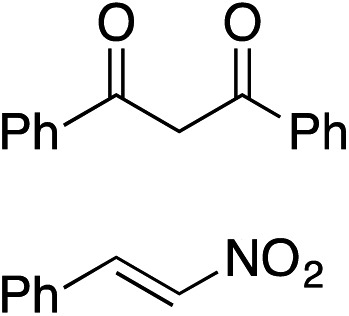
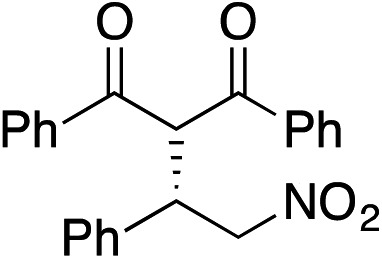
| 3 |
|
|
81% (91 : 9) | 90% (10 : 90) | 89% (95 : 5)10f,h |
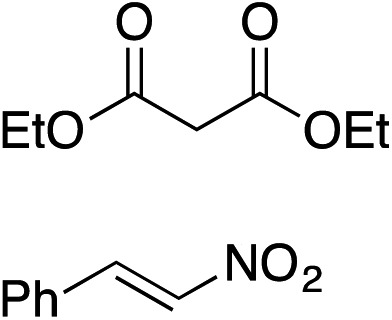
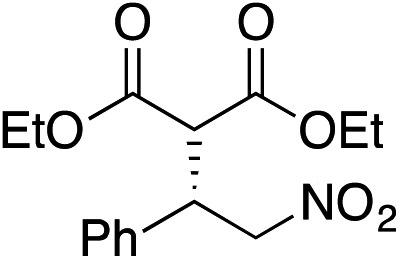
| 4 |
|
|
88% (89 : 11) | 70% (20 : 80) | 83% (95 : 5)10h |
| 5 |
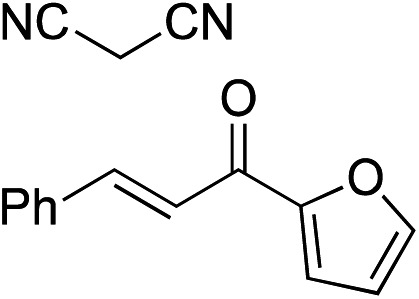
|
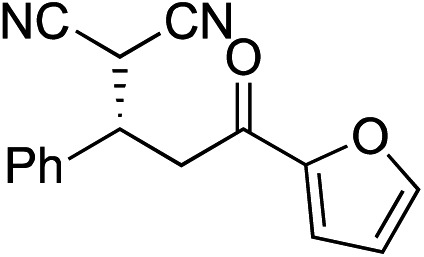
|
95% (95 : 5) | 93% (7 : 93) | 96% (93 : 7)10e |
| 6 |
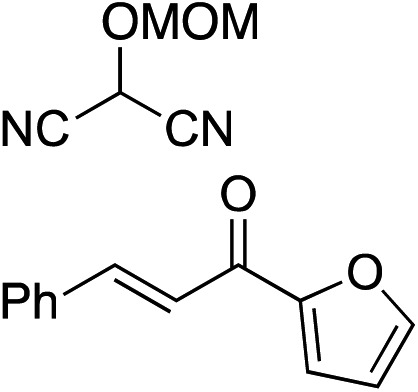
|
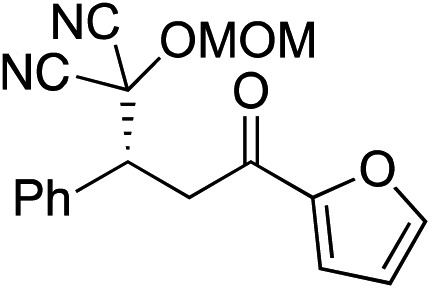
|
95% (87 : 13) | 95% (16 : 84) | — |
aProducts of catalyst 1 depicted.
bCatalyst structures and reaction conditions are shown in Table S3, ESI. MOM = methoxymethyl. In all cases catalyst 1 was used as 98 : 2 E : Z and catalyst 2 as 1 : 99 E : Z.
