Figure 4.
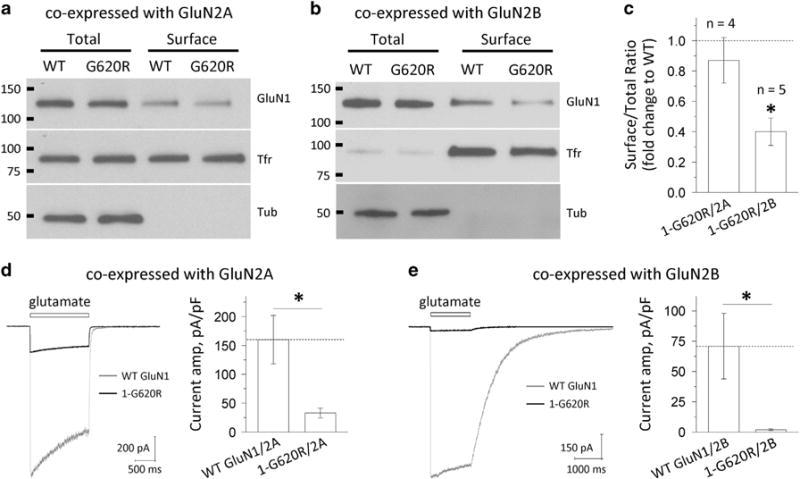
GluN1-G620R alters N-methyl-D-aspartate (NMDA) receptor surface expression and current response. (a and b) The surface proteins (wild-type GluN1 or GluN1-G620R) were expressed in HEK293 cells for 24 h, labeled with biotin and pulled down with avidin beads. Equivalent amounts of wild type and G620R protein samples were separated by SDS-polyacrylamide gel electrophoresis (PAGE; GluN2A: 10 μl of total and surface; GluN2B: 5 μl of total and 30 μl of surface). The expression of NMDARs was assessed by western blotting for GluN1, transferrin receptor (TfR) and tubulin (Tub). Total GluN1 signals were normalized to tubulin signal, which served as a loading control. TfR served as a control to show relatively equal surface labeling. Representative blots are shown for HEK293 cells expressing wild-type GluN1/GluN2A and GluN1-G620R/GluN2A (a), and GluN1/GluN2B and GluN1-G620R/GluN2B (b). (c) Densitometry was used for chemiluminescence signal quantification, and the ratio of surface-to-total protein level of wild-type GluN1 was compared to GluN1-G620R by paired t-tests for GluN2A (P = 0.43, n = 4) and GluN2B (*P = 0.0001, n = 5). Mutant surface-to-total protein ratios were normalized to the wild type (dashed line) and plotted as mean ± s.e.m. (d and e) Current response was recorded by using the whole-cell voltage patch-clamp recordings from transiently transfected HEK293 cells at holding potential of −60 mV. The current amplitude normalized to cell capacitance was significant decreased in the GluN1-G620R mutant when co-expressed with GluN2A (d, 33 pA/pF vs 160 pA/pF for the wild type, n = 7, 13; *P = 0.001) or with GluN2B (e, 2.0 pA/pF vs 71 pA/pF for the wild type, n = 5, 6; *P = 0.02).
