Figure 3.
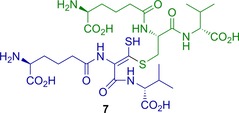
Proposed structure for 7 as assigned by NMR and MS analyses. (S)‐2‐Amino‐6‐(((S)‐3‐(((E)‐2‐((S)‐5‐amino‐5‐carboxypentanamido)‐3‐(((R)‐1‐carboxy‐2‐methylpropyl)amino)‐1‐mercapto‐3‐oxoprop‐1‐en‐1‐yl)thio)‐1‐(((R)‐1‐carboxy‐2‐methylpropyl)amino)‐1‐oxopropan‐2‐yl)amino)‐6‐oxohexanoic acid 7 is an oxidised dimer of two molecules of ACV 1: one ACV (green) is intact (but for its thiol S−H), the other (blue) has been doubly oxidised at cysteine (from thiol to thiocarboxylic acid oxidation state) and contains an α,β‐desaturated cysteinyl residue. Note that the E/Z stereochemistry of the alkene in 7 (and analogous products) is not defined by the NMR analyses and may equilibrate through tautomerism. Stereochemistry at all other positions is assumed to be as in ACV 1.
