Abstract
Corneal wound healing is a complex process that occurs in response to various injuries and commonly used refractive surgery. It is a significant clinical problem, which may lead to serious complications due to either incomplete (epithelial) or excessive (stromal) healing. Epithelial stem cells clearly play a role in this process, whereas the contribution of stromal and endothelial progenitors is less well studied. The available evidence on stem cell participation in corneal wound healing is reviewed, together with the data on the use of corneal and non‐corneal stem cells to facilitate this process in diseased or postsurgical conditions. Important aspects of corneal stem cell generation from alternative cell sources, including pluripotent stem cells, for possible transplantation upon corneal injuries or in disease conditions are also presented. Stem Cells 2017;35:2105–2114
Keywords: Corneal epithelium, Keratocyte, Corneal endothelium, Wound healing, Gene therapy, Stem cell, Pluripotent stem cell, Cell transplantation
Significance Statement.
This is the first review directly addressing the role of various stem cells in corneal wound healing. The significance is that, in contrast with most other reviews, it covers all major corneal cell types in a comprehensive way, showing similarities and differences in the healing process and the usage of stem cells for therapy. Potential gaps in knowledge and future directions are specifically delineated.
Introduction
As the outermost part of the eye, cornea is directly exposed to the environment and is thus prone to potential injuries due to burns, abrasions, contact lens problems, insufficient tear production, infections and other disease conditions, as well as refractive surgeries. In many cases, such injuries cause wounds triggering the healing process in the tissue. Corneal wound healing is thus not only a basic science topic but is also a significant clinical concern. Cornea has three main cell types, the stratified surface epithelium, the stromal keratocytes, and the innermost single‐layered endothelial cells, which are actually neuroepithelial in nature. These cells have similarities and differences in ways and mechanisms by which they heal wounds 1. Similarities include cell migration and proliferation, growth factor and cytokine involvement, and reorganization of the extracellular matrix (ECM). Differences are related to specific behavior of healing cells. The epithelial cells migrate as a sheet and may proliferate in the process that involves peripheral stem cells, undergoing differentiation and stratification after closure of the defect. Epithelial wounds are also accompanied by apoptosis of stromal keratocytes under the wound caused by the epithelial interleukin‐1. These keratocytes are gradually replaced by live cells usually without scarring. During healing of stromal wounds caused by injury or refractive surgery, quiescent keratocytes undergo transformation to activated fibroblasts and α‐smooth muscle actin‐containing myofibroblasts, with participation of both resident and circulating immune cells. This process involves transforming growth factor (TGF)‐β and may be deregulated, leaving a stromal scar or haze due to excessive ECM deposition and hypercellularity. The corneal endothelium largely heals through migration and spreading, with documented TGF‐β driven epithelial‐mesenchymal transformation, whereas cell proliferation is less important. These cell type‐dependent wound healing events are summarized in Figure 1. The corneal epithelial stem cells have been convincingly shown to participate in wound healing, but the contribution of stromal and endothelial stem cells to this process is still debatable. In this review, we will analyze recent data on the identification of corneal stem cells, their possible roles in wound healing, and existing and future possibilities for using both autologous and allogeneic stem cell therapies.
Figure 1.
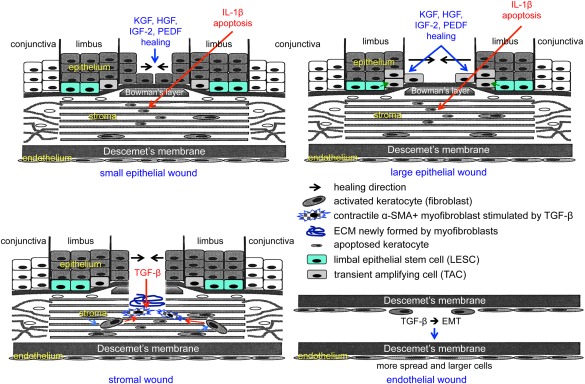
Schematic representation of main events during corneal epithelial, stromal, and endothelial wound healing. Top left, healing of small epithelial wound under the influence of several growth factors entails participation of central cells only. Keratocytes under the wound die by apoptosis mediated by epithelium‐derived interleukin‐1β. Top right, healing of large epithelial wound under the influence of several growth factors entails participation of both limbal epithelial stem cells and their progeny (transient amplifying cells), as well as of central cells. Bottom left, healing of a stromal wound entails activation of keratocytes to form fibroblasts that are transformed to motile myofibroblasts under the influence of transforming growth factor (TGF)‐β. Myofibroblasts positive for α‐smooth muscle actin contract the wound, and also produce and remodel the extracellular matrix in the wound bed. Burns are also associated with stromal neovascularization (not shown). Bottom right, healing of endothelial wound entails epithelial–mesenchymal transformation (EMT) and cell migration under the influence of TGF‐β. Wound closure is accompanied by increased spreading and enlargement of endothelial cells that undergo the process opposite to EMT, that is, mesenchymal–epithelial transformation. Abbreviations: ECM, extracellular matrix; EMT, epithelial–mesenchymal transformation; HGF, hepatocyte growth factor; IGF‐2, insulin‐like growth factor‐2; IL, interleukin; KGF, keratinocyte growth factor; PEDF, pigment epithelium‐derived factor; TGF, transforming growth factor; α‐SMA, α‐smooth muscle actin.
Stem Cells for Epithelial Wound Healing
Limbal Epithelial Stem Cells in Wound Healing
Corneal epithelium comprises a single layer of basal cells and 4–6 layers of stratified squamous epithelial cells, which are continuously shed and replenished in corneal homeostasis. This cell turnover helps to maintain a uniform structure and thickness avoiding loss of corneal transparency. Corneal epithelial renewal depends on adult limbal epithelial stem cells (LESCs) located at the periphery of the corneoscleral junction, limbus (Fig. 1) 2, 3. LESC are quiescent cells located in the basal layer of the limbal epithelium in a specific structured niche called palisades of Vogt, and/or in the deeper limbal epithelial crypts and focal stromal projections 4, 5, 6. LESC have been localized and tentatively identified based on their colony‐forming ability, proliferative potential, slow cycling nature (BrdU or EdU label‐retaining cells), expression of specific antigens, and lack of terminal differentiation markers 1, 7, 8. Loss of LESC leads to limbal stem cell deficiency (LSCD) that may be due to mechanical, chemical, and thermal injuries, genetic defects or chronic disease, leading to conjunctival ingrowth with neovascularization, corneal opacity, and vision loss 9. LSCD is usually treated clinically by transplantation of an autologous or allogeneic limbal graft or cultured LESC 10, 11, 12, 13, 14, 15.
During corneal homeostasis and wound healing, LESC proliferate and give rise to transient amplifying cells (TACs) that further divide and differentiate and migrate to the center of the cornea (Figs. 2; 3, left) to regenerate the epithelial layers 8, 12, 13, 14, 15, 16. In vivo multicolor lineage tracing of keratin 14 (K14)‐positive cells during closure of large wounds (Fig. 3, right) demonstrated centripetal miration of individual limbal cells as radial streaks to the center of the cornea 16, 17, 18. Healing of small epithelial wounds may be achieved by central epithelial cells 19, 20. After corneal injury with large wounds, limbal stem cell activation leading to healing occurs, which is mediated by environmental cues such as growth factors, cytokines, ECM, and integrin receptors 1, 17, 21, 22, 23, 24. Growth factor systems activating LESC upon corneal epithelial damage include among others keratinocyte growth factor in limbal fibroblasts and its receptor in the epithelial cells, insulin‐like growth factor‐1 and ‐2 and their receptors, and pigment epithelium‐derived factor 23, 25, 26, 27. A neuroprotective cytokine, ciliary neurotrophic factor, was shown to activate LESC in both normal and diabetic mouse corneal epithelial wound healing 28, 29. A Rho‐associated protein kinase (ROCK) inhibitor Y‐27632 promotes limbal epithelial cell proliferation in vitro and wound healing in vivo 30. The effects of Y‐27632 may be due to the suppression of Smad2 expression 31, thus interfering with TGF‐β signaling activation that delays re‐epithelialization 32. ROCK inhibitor can also block apoptosis by downregulating caspase‐10 and ‐3 33.
Figure 2.
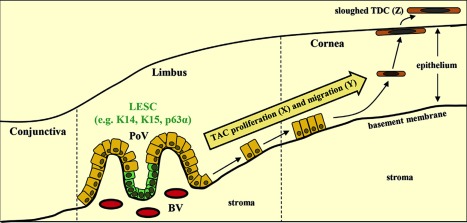
Corneal epithelial cell maintenance by limbal epithelial stem cell (LESC). LESC (expressing K14, K15, and p63α, and potentially other markers) residing in the basal epithelium of the palisades of Vogt, divide (X) and differentiate into transient amplifying cells while they migrate centripetally (Y), first horizontally along the basement membrane then diagonally through the epithelial tiers, before reaching the superficial epithelium in the central cornea as terminally differentiated cells that are sloughed from the ocular surface (Z). According to Thoft and Friend, X + Y = Z. Reproduced with permission from 8. Abbreviations: BV, blood vessel; LESC, limbal epithelial stem cell; PoV, palisades of Vogt; TAC, transient amplifying cell; TDC, terminally differentiated cell.
Figure 3.
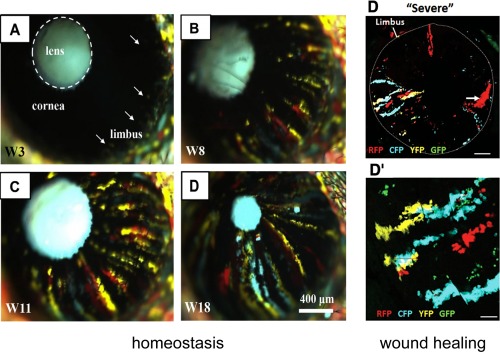
Clonal expansion and participation of limbal epithelial stem cell in corneal epithelial homeostasis and wound healing. Left, 6‐week old transgenic mice were injected intraperetoneally with tamoxifen over 3 consecutive days. Mice were monitored long‐term by intravital microscopy, as described previously. Colored patches were observed in the peripheral cornea at 3 weeks (W) post‐tamoxifen (A, arrows), which developed into discrete stripes (B, C; 8 and 11 weeks post‐tamoxifen, respectively) migrating toward the central cornea intersecting the apex by 18 weeks (D). Notably, the intraocular lens autofluoresces and the eyelid skin recombined within 1 week of tamoxifen treatment. Scale bar = 400 μm. Reproduced with permission from 8. Right, expansion and migration of K14+ fluorescent clones in Confetti transgenic mice during wound healing. Chemical burns to the corneal surface were achieved by topical application of dimethyl sulfoxide (DMSO) combined with tamoxifen induction. (D): Repeated DMSO application for 3 successive days caused a “severe” wound to the cornea. One week following the last DMSO treatment, multiple wide streaks of fluorescent cells were observed; (D′) is a magnification of (D). Limbal–corneal border is annotated by a dashed line. Scale bar = 500 μm (D); = 75 μm (D′). Reproduced with permission from 17. Abbreviations: CFP, cyan fluorescent protein; GFP, green fluorescent protein; RFP, red fluorescent protein; YFP, yellow fluorescent protein.
Our data show that in human diabetic corneas hepatocyte growth factor (HGF) receptor, c‐met, plays a role in LESC activation and epithelial wound healing. In these corneas, HGF is upregulated, but c‐met is downregulated. Corneas from long‐term diabetics have dysfunctional LESC with significantly lower than normal expression of several putative stem cell markers 34. Restoring c‐met levels by gene therapy normalized delayed wound healing and significantly increased LESC marker expression in human diabetic organ‐cultured corneas and primary LESC‐enriched cultures 34, 35, 36. Similar normalization was observed upon short hairpin RNA‐mediated inhibition of proteinases matrix metalloproteinase‐10 and cathepsin F that are upregulated in diabetic corneas 35, 36. Corneal epithelial wound healing thus involves LESC and may be compromised in disease conditions such as diabetes. Therefore, gene and cell therapeutic approaches may help achieve faster wound closure and minimize complications.
Stem Cell Therapy
In various pathological conditions, such as hereditary disorders (e.g., aniridia or Stevens–Johnson syndrome), burns, diabetes, infections, and chronic inflammation, LESC damage or limbal niche disruption occurs leading to partial or total LSCD that seriously compromises epithelial regeneration and wound healing 1, 12, 14, 15, 35, 37, 38. Therefore, limbal cell transplantation is now regarded as a promising therapeutic approach to restore the stem cell loss and function in LSCD of various etiologies 12, 14.
Thermal and especially chemical corneal burns represent the major clinical indication for transplanting LESC to improve compromised epithelial wound healing 12, 15, 39, 40, 41. The most common alkaline burns cause necrosis of the corneal epithelium with partial or complete LSCD, dissolution of stromal collagen, significant inflammation that does not resolve until the epithelial defect is closed, and a later neovascularization brought about by invading conjunctiva 19. Since the pioneering work of Barraquer 11, transplantation of limbal epithelium enriched in LESC is clinically used for corneal burns to achieve re‐epithelialization, decrease inflammatory cell immigration, and suppress neovascularization. These impressive effects were very similar in a number of animal studies and clinical interventions involving hundreds of treated patients to date. The rates of clinical success are variable depending on the burn severity and are measured either as improved corneal conditions and visual acuity, or epithelialized, clear, and avascular cornea. After 1 year, complete or partial success in treating corneal burns with limbal cell transplantation was achieved in 75%–81% of adult patients; after 3 years, it was around 70% 12, 15, 37, 38, 40, 42. The use of autologous and allogeneic LESC produced similar clinical results, although in the latter case patients received immunosuppressive drugs 12, 43.
LESC for LSCD are transplanted as autologous and allogeneic biopsies of the limbal tissue or as culture‐expanded cells 10, 11, 12, 15, 38, 40, 41. The prevailing methods used clinically differ depending on regulatory standards in a specific country. In the U.S., biopsy‐based keratolimbal transplantation is still the only method allowed by the Food and Drug Administration, whereas in Europe, Japan, and especially in India, cell culture‐based transplants are becoming very common.
The conjunctival limbal autografts (transplantation of a small biopsied piece of limbal tissue from a healthy eye to the region with LSCD in the fellow eye of the same patient) were first introduced for unilateral burn victims over 50 years ago by Barraquer 11 and remain the Food and Drug Administration‐approved method of choice in the U.S. Allografts are also used, although they require long‐term immunosuppression 10, 12, 14, 38. For eyes with complete LESC failure, primarily due to burns, allogeneic penetrating limbo‐keratoplasty has been developed. The procedure consists of the transplantation of the central corneal button with LESC region by means of eccentric trephination of the donor cornea 41. Autologous biopsy‐based LESC grafting has a significant drawback related to the need of relatively large pieces of limbal tissue. For this reason, it should be used with caution because of a possibility of iatrogenic LSCD, as the LESC never regenerate at the biopsy site 15. Bilateral LSCD calls for allogeneic tissue use requiring immunosuppression. The biopsy‐based allogeneic grafts for corneal burns have a median survival of 3.4 years, requiring subsequent retransplantation 41.
Another way of managing LSCD, introduced in 1997 by Pellegrini's group, is the transplantation of LESC‐enriched cell cultures expanded in vitro 10, 37, 38. This procedure has now become a treatment of choice for LSCD in many countries 12, 15, 38, 42, 44, 45. Recently, the first stem cell‐based treatment Holoclar was granted license in the European Union for the management of moderate to severe LSCD in adults. The treatment consists of transplanting culture‐expanded autologous limbal epithelial cells on a fibrin support 46. Limbal cultures for clinical use are composed of stem cells and their progeny (TACs). Clonogenic assays in these cultures suggest a minor proportion of LESC, but they still can replace the damaged limbal epithelium and repopulate the corneal surface 37. A comparison of transplantation results of cultured LESC versus conjunctival‐limbal autografts for severe unilateral burns showed very similar success rates at 6 months post surgery. However, the amount of tissue required for cultured LESC expansion is minimal, making this procedure preferable 47. The similarity of success rates upon transplantation of limbal grafts or cultured LESC was confirmed in other studies 12, 13. Cultured LESC may be used autologously in “simple limbal epithelial transplantation” when small biopsies of limbal tissue are expanded, and the resultant cultures are used to treat burns to ensure epithelial wound healing 15, 42, 45, 47. Allogeneic LESC cultures have also been used successfully. Interestingly, cryopreserved and expanded LESC cultures lose immunogenicity by downregulating major histocompatibility complex (MHC) protein expression, making immune rejection less of a problem 48. Historically, LESC were expanded for transplantation using fetal bovine serum in the medium and mouse 3T3 cells as a feeder layer 10. More recently, xenobiotic‐free cultures were developed using a feeder‐free system, and either serum‐free or human serum‐supplemented media to comply with rigorous regulatory requirements 49, 50, 51, 52.
For easier handling of cells during transplantation, LESC are cultured on various biological supports. The cells on a support are sutured or glued to the limbal area of a damaged eye, and the cells migrate over time to the denuded area to heal the wound (Fig. 4; 15). Some LESC supports are regularly used in the clinic, such as human amniotic membrane (HAM) or fibrin gel 15, 46. A number of other supports are in preclinical development and may even offer better standardization and reproducibility than the clinically used ones 53. Most often, cells are cultured on denuded HAM 12, 15, 42, 54, which is essentially a basement membrane with a composition similar to the limbal epithelial basement membrane 55. For this reason, it may also provide the cells with the correct ECM that is an important part of the limbal stem cell niche 39, 56. In addition to providing good support for cells, HAM supplies some growth factors and is non‐immunogenic and anti‐inflammatory. However, HAM needs to be thoroughly screened for infections and communicative diseases, and properly stored in sterile conditions 57. Fibrin is used as LESC support in the Holoclar system approved for clinical use in Europe. Cells usually grow well on fibrin, but it may need to be stabilized against degradation 58. It may also reduce cell migration, which should be taken into account when using it for LESC transplantation to heal corneal burn wounds 59. It should be noted that growing cells on thermosensitive plates, from which they detach as sheets at room temperature, allows for transplanting them without a support/carrier and sutures. This was tried clinically on a small number of patients with LSCD using an alternative cell source from oral mucosa. Upon transplantation, all four patients retained clear corneas for the 14‐month follow‐up period 60.
Figure 4.

Re‐epithelialization of the corneal surface by human amniotic membrane (HAM)‐grown limbal epithelial stem cell‐enriched limbal cultures. Left, low magnification showing HAM with attached limbal cells placed on top of human organ cultured cornea that has been de‐epithelialized by mild NaOH treatment. HAM is secured to the corneal surface by derma + flex gel adhesive (formulated medical cyanoacrylate from Chemence Medical, Alpharetta, GA). Scale bar = 100 μm. Right, high magnification showing limbal cells that have migrated from HAM and repopulated the corneal surface. Scale bar = 40 μm. Abbreviation: HAM, human amniotic membrane.
A controversy recently emerged concerning the survival of transplanted LESC. In some studies, DNA analysis did not find transplanted allogeneic cells (either as cultures or as limbal grafts) after 3–9 months. At the same time, the corneal surface remained stable 38, 61. In other clinical studies, however, grafts survived for 3–8 years before being rejected, attesting to the ability of transplanted cells to exist for a long time 15, 62. It is unclear what cells assumed the limbal function in cases where donor cells disappeared early, and whether this may also happen with autologous transplants. In any event, if the LESC grafts eventually fail, repeat transplants can be made successfully, as a study in burn patients has shown 63.
Drawbacks of transplanting LESC‐enriched cultures include a limit of cell passage number, increased risk of allograft rejection and disease transmission, and potential gene and/or cell contamination from mouse 3T3 cell feeder 64. Many countries also face a shortage of donor corneas limiting allogeneic LESC transplantation in bilateral LSCD. For these reasons, alternative cell sources able to differentiate into the corneal epithelium have been tested for restoration of the corneal surface and wound healing. These include cultured oral mucosal epithelium, hair follicle, conjunctival and epidermal epithelium, amniotic epithelial cells, umbilical cord lining epithelial cells, as well as mesenchymal stem cells (MSCs) from adipose tissue, bone marrow, orbital fat, and immature dental pulp 13, 14, 22, 65, 66, 67. Cultured autologous oral mucosal epithelium has been used clinically; again, most frequently, for chemical burns, although with somewhat lower success rates than with LESC transplantation (close to 70%) 13, 14, 65, 66. Most other cell types have only been examined in preclinical models 22, 67.
Embryonic stem cells (ESC) and induced pluripotent stem cells (iPSC) that are renewable, easily expandable and bankable are emerging as an attractive source for stem cell‐based therapy in diseased or severely wounded cornea 68. They can be directed to limbal differentiation with some degree of success, but this process is not yet fully optimized 68, 69, 70, 71. The use of combined soluble factors for specific time periods and proper extracellular support appears to be critical for achieving reliable limbal epithelial differentiation. Before the introduction of these cell sources into clinical practice certain critical issues need to be resolved, such as the risk of mutagenesis and tumorigenesis, high cost of the process, and reproducibility of differentiation in different ESC or iPSC clones 39.
In summary, the therapeutic potential of LESC biopsies or cultures is evident from ample clinical data on transplantation and wound healing, particularly in cases of severe LSCD caused by burns. Optimization of LESC cultures, and/or standardization of directed limbal differentiation of iPSC are important for the expansion of autologous stem cell‐based therapy in corneal diseases associated with LESC damage. The use of various MSCs may also be advantageous due to autologous nature and ability to repair corneas after burns. However, potential problems related to cell support standardization and graft longevity need to be explored further.
Stem Cells for Stromal Wound Healing
Human limbal stroma contains cells that express Pax6 and ABCG2 progenitor markers and can be induced by fibroblast growth factor‐2 to differentiate into corneal keratocytes in vitro. These cells also express markers of MSCs and can differentiate into non‐keratocyte lineages in special culture media 72. Hopkinson's group has found that such stromal stem cells from human limbus were multipotent and fulfilled the criteria for MSC developed by the International Society of Cellular Therapy 73. Very similar MSCs have been recently isolated from human central corneal stroma as well 74. Additionally, monocytic progenitor cells expressing CD133 marker and capable of keratocytic differentiation could be isolated as a side population from the stroma of donor human corneas and successfully differentiated into lumican‐expressing keratocytes 75. Corneal stromal stem cells have been already used for stromal engineering in vitro 72. Importantly, injection of isolated human stromal stem cells restored stromal transparency in a lumican‐null mouse model with corneal opacity 72.
In animal corneal wound models, the injection of human stromal stem cells from limbal biopsies prevented fibrotic scar formation (Fig. 5) and contributed to the normal regeneration of stromal ECM with the structure similar to uninjured corneas 76. These limbal biopsy‐derived stromal cells inhibited neutrophil migration into the wounded stroma, suppressing fibrotic tissue deposition 77. Such cells also contributed to increased strength of adhesion of flaps created in the laser‐assisted in situ keratomileusis procedure in organ‐cultured corneas, which may be clinically important for flap integrity 78. Additional to these endogenous cells, various MSCs in vivo significantly reduced stromal neovascularization in alkaline burn models and diminished corneal opacity and inflammation upon penetrating injury 79, 80.
Figure 5.
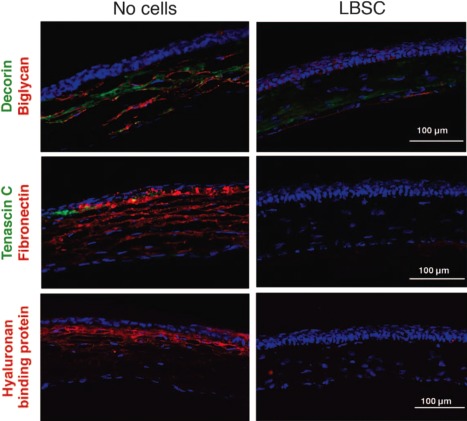
Debridement‐wounded mouse corneas were treated with fibrin gel only (no cells) or with 50,000 limbal biopsy‐derived stromal cells in fibrin gel. After 4 weeks of healing, histological sections (epithelium oriented up) were stained for fibrotic markers decorin, biglycan, tenascin C, fibronectin, and hyaluronan binding protein. Images are representative of sections from three corneas for each condition. Note lack of fibrotic proteins from the stem cell‐treated corneas that are now similar to the untreated ones. Reproduced with permission from 76. Abbreviation: LBSC, limbal biopsy‐derived stromal cell.
In summary, corneal stromal stem cells and other MSCs appear to be a valuable tool for the treatment of burns and reduction of stromal fibrosis and opacity due to penetrating wounds in animal studies. These promising data should form the basis of the future clinical trials. The availability of iPSC‐derived keratocytes 81 may eventually provide alternative stable sources for future stromal cell transplantation.
Stem Cells for Endothelial Repair and Wound Healing
Corneal endothelial cells (CEC) appear to close the wound mainly by migration and enhanced spreading, whereas cell proliferation plays a secondary role. Human CEC can hardly be expanded either in vivo or in vitro 82. The existence of human corneal endothelial stem cells has not yet been conclusively established. Earlier studies used neurosphere‐forming cell isolation and reported increased numbers of spheres from the endothelial periphery 83. Expanded cells could be incorporated into the endothelial layer in a model of CEC deficiency 83. Slow cycling endothelial cells and small cells with high nuclear‐cytoplasmic ratio were also observed at the corneal periphery. They expressed stem cell markers Oct3/4, Wnt‐1, Pax6, and Sox2 and had elevated telomerase activity. As such, they were postulated to be the endothelial progenitors capable of proliferation and expansion during wound healing 84, 85.
Recent studies addressed the possible application of other stem cells to enhance endothelial repair and wound healing. MSCs from umbilical cord blood were shown to engraft into wounded cultured endothelial sheets in vitro and acquire an endothelial phenotype 86. As an alternative source for CEC transplantation, such cells can be obtained by in vitro differentiation of human pluripotent stem cells. CECs are derived from neural crest stem cells (NCSC), which may allow using NCSC to generate autologous CEC substitutes. The differentiation of rodent NCSC into functional CEC that covered the Descemet's membrane after transplantation was reported in the rat model of corneal endothelial deficiency 87. Similar to the epithelial cells, ROCK inhibitor also enhanced CEC proliferation and migration in the in vitro and in vivo models of corneal endothelial wound healing 88, suggesting its potential therapeutic use. CEC were also generated from ESC and iPSC by a two‐stage protocol 89 or directly by suppressing TGF‐β and ROCK signaling 90. Functional CEC‐like cells derived from ESC restored transparency when transplanted into the eyes of rabbits with CEC dysfunction 91. Such cells had high expression of CEC markers AQP1, Na+‐K+‐ATPase, type VIII collagen, and ZO‐1, and a gene profile similar to CEC 92, 93.
In summary, a significant progress has been achieved in producing CEC from human ESC/iPSC‐derived NCSC in an in vitro two‐step process. This approach offers promise for the development of endothelial replacements suitable to treat corneal edema due to pathological CEC dysfunction (e.g., in Fuchs’ endothelial corneal dystrophy) and wound‐related complications of corneal surgeries.
Conclusion
Delayed, incomplete, or excessive corneal wound healing remains a significant clinical concern calling for the development of new efficient therapeutics. It is advantageous to accelerate epithelial wound healing, especially in conditions when it is slow or incomplete, such as in diabetes. During stromal healing, the treatment should counteract excessive tissue remodeling, which may lead to scarring and haze. During endothelial healing, a problem of low cell proliferative potential and possible complications from epithelial–mesenchymal transformation should be circumvented. In recent years, stem cell transplantation has emerged as an effective tool that may be able to fine tune wound healing and improve it for the patient's benefit. Clinical success in normal healing restoration has been achieved mainly for epithelial cells with the advent of cultured LESC transplantation for LSCD caused by various burns. This technique has proven to be as efficient as the previously introduced biopsy‐based keratolimbal transplantation. At the same time, transplanted limbal cells need be further studied in regard to the factors that influence LESC‐mediated healing, its molecular mechanisms, the duration of the transplant effect, and the longevity of the grafted cells 15, 38. Organ‐cultured human corneas could be used for this purpose, as animal data may not be fully relevant to the human conditions. There are some promising data using animal models on the reduction of scarring and haze by limbal stromal stem cells, but these results need to be confirmed with large animal models before their translation into clinical practice, in accordance with regulatory requirements. For the endothelial cells, there is clear need for more data on the identification of resident stem cells and their transplantability. In all three areas, alternative sources of stem cells for possible transplantation have been identified including various epithelial progenitors and MSCs. These important studies need to be expanded to streamline and standardize protocols for autologous non‐corneal cell transplantations, especially in cases when the patient's respective corneal cells are not available.
This decade is seeing an exciting surge of studies using differentiation of ESC and iPSC into corneal cells, and some promising candidates may soon enter clinical trials. The resultant corneal epithelial cells may be closer to clinical use, whereas stromal and endothelial cells need to be generated from pluripotent stem cells with better reliability. ESC‐ or iPSC‐derived differentiated cells offer the advantages of an autologous source, easy expansion and banking, but the safety issues including mutagenicity and tumorigenicity must be carefully addressed before clinical translation 39. Another promising approach to consider and develop is the genetic manipulation of cultured stem cells in disease conditions, such as diabetes, where stem cell dysfunction may be reverted by a specific gain‐of‐function and/or loss‐of‐function gene therapy 36. In the near future, viral gene therapy of stem cells may be complemented by nano vehicle‐driven therapeutics. Finally, new gene editing techniques, such as CRISPR/Cas9, could also be applied to diseased/dysfunctional corneal stem cells to precisely and stably regulate gene expression. Such techniques may also be considered for targeting MHC proteins to decrease cell immunogenicity 94 to expand and facilitate the usage of allogeneic stem cell transplantation.
In conclusion, the available data show the importance of stem cells in corneal epithelial, stromal, and endothelial wound healing in disease, injury, or postsurgical conditions. Corneal epithelial stem cells transplantation has been successfully used in clinic to ensure healing upon serious injuries including burns, and preclinical data suggest similar benefits of stromal and endothelial stem cells. The field has expanded to include various non‐corneal sources where the patient's corneal cells are not available, with ESC‐ and iPSC‐derived limbal cells showing promise for future transplantation upon corneal injuries. The advances in transplant techniques and the range of available cell sources that can be used to optimize the treatment of aberrant corneal wound healing can give reassurance to patients with corneal injuries that preserving vision may be possible in the near future.
Author Contributions
M.S. and A.A.K.: manuscript writing, editing, and final approval; C.N.S.: manuscript editing, and final approval, financial support, and administrative support; A.V.L.: conception, manuscript writing, editing, and final approval.
Disclosure of Potential Conflicts of Interest
The authors indicated no potential conflicts of interest.
Acknowledgments
This work was supported by NIH grants R01 EY13431, R01 EY023429 (to A.V.L.), R01 EY025377 (to M.S.), and Board of Governors Regenerative Medicine Institute grants. The authors thank Dr. Soshana Svendsen for constructive editing of the manuscript.
References
- 1. Ljubimov AV, Saghizadeh M. Progress in corneal wound healing. Prog Retin Eye Res 2015;49:17–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Davanger M, Evensen A. Role of the pericorneal papillary structure in renewal of corneal epithelium. Nature 1971;229:560–561. [DOI] [PubMed] [Google Scholar]
- 3. Tseng SC. Regulation and clinical implications of corneal epithelial stem cells. Mol Biol Rep 1996;23:47–58. [DOI] [PubMed] [Google Scholar]
- 4. Dua HS, Shanmuganathan VA, Powell‐Richards AO et al. Limbal epithelial crypts: A novel anatomical structure and a putative limbal stem cell niche. Br J Ophthalmol 2005;89:529–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Shortt AJ, Secker GA, Munro PM et al. Characterization of the limbal epithelial stem cell niche: Novel imaging techniques permit in vivo observation and targeted biopsy of limbal epithelial stem cells. Stem Cells 2007;25:1402–1409. [DOI] [PubMed] [Google Scholar]
- 6. Molvaer RK, Andreasen A, Heegaard S et al. Interactive 3D computer model of the human corneolimbal region: Crypts, projections and stem cells. Acta Ophthalmol 2013;91:457–462. [DOI] [PubMed] [Google Scholar]
- 7. Sun TT, Lavker RM. Corneal epithelial stem cells: Past, present, and future. J Invest Dermatol Symp Proc 2004;9:202–207. [DOI] [PubMed] [Google Scholar]
- 8. Richardson A, Wakefield D, Di Girolamo N. Fate mapping mammalian corneal epithelia. Ocul Surf 2016;14:82–99. [DOI] [PubMed] [Google Scholar]
- 9. Biber JM, Holland EJ, Neff KD. Management of ocular stem cell disease. Int Ophthalmol Clin 2010;50:25–34. [DOI] [PubMed] [Google Scholar]
- 10. Pellegrini G, Traverso CE, Franzi AT et al. Long‐term restoration of damaged corneal surfaces with autologous cultivated corneal epithelium. Lancet 1997;349:990–993. [DOI] [PubMed] [Google Scholar]
- 11. Barraquer J. Panel three discussion In: King JH, McTigue JW, eds. The Cornea World Congress. Washington: Butterworths, 1965:354. [Google Scholar]
- 12. Baylis O, Figueiredo F, Henein C et al. 13 years of cultured limbal epithelial cell therapy: A review of the outcomes. J Cell Biochem 2011;112:993–1002. [DOI] [PubMed] [Google Scholar]
- 13. Osei‐Bempong C, Figueiredo FC, Lako M. The limbal epithelium of the eye—A review of limbal stem cell biology, disease and treatment. Bioessays 2013;35:211–219. [DOI] [PubMed] [Google Scholar]
- 14. Atallah MR, Palioura S, Perez VL et al. Limbal stem cell transplantation: Current perspectives. Clin Ophthalmol 2016;10:593–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sangwan VS, Sharp JAH. Simple limbal epithelial transplantation. Curr Opin Ophthalmol 2017;28:382–386. [DOI] [PubMed] [Google Scholar]
- 16. Di Girolamo N, Bobba S, Raviraj V et al. Tracing the fate of limbal epithelial progenitor cells in the murine cornea. Stem Cells 2015;33:157–169. [DOI] [PubMed] [Google Scholar]
- 17. Amitai‐Lange A, Altshuler A, Bubley J et al. Lineage tracing of stem and progenitor cells of the murine corneal epithelium. Stem Cells 2015;33:230–239. [DOI] [PubMed] [Google Scholar]
- 18. Dorà NJ, Hill RE, Collinson JM et al. Lineage tracing in the adult mouse corneal epithelium supports the limbal epithelial stem cell hypothesis with intermittent periods of stem cell quiescence. Stem Cell Res 2015;15:665–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wagoner MD. Chemical injuries of the eye: Current concepts in pathophysiology and therapy. Surv Ophthalmol 1997;41:275–313. [DOI] [PubMed] [Google Scholar]
- 20. Chang CY, Green CR, McGhee CN et al. Acute wound healing in the human central corneal epithelium appears to be independent of limbal stem cell influence. Invest Ophthalmol Vis Sci 2008;49:5279–5286. [DOI] [PubMed] [Google Scholar]
- 21. Li Z, Burns AR, Rumbaut RE et al. T cells are necessary for platelet and neutrophil accumulation in limbal vessels and efficient epithelial repair after corneal abrasion. Am J Pathol 2007;171:838–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Blazejewska EA, Schlötzer‐Schrehardt U, Zenke M et al. Corneal limbal microenvironment can induce transdifferentiation of hair follicle stem cells into corneal epithelial‐like cells. Stem Cells 2009;27:642–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Trosan P, Svobodova E, Chudickova M et al. The key role of insulin‐like growth factor I in limbal stem cell differentiation and the corneal wound‐healing process. Stem Cells Dev 2012;21:3341–3350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Castro‐Muñozledo F. Review: Corneal epithelial stem cells, their niche and wound healing. Mol Vis 2013;24:1600–1613. [PMC free article] [PubMed] [Google Scholar]
- 25. Cheng CC, Wang DY, Kao MH et al. The growth‐promoting effect of KGF on limbal epithelial cells is mediated by upregulation of ΔNp63α through the p38 pathway. J Cell Sci 2009;122:4473–4480. [DOI] [PubMed] [Google Scholar]
- 26. Jiang Y, Ju Z, Zhang J et al. Effects of insulin‐like growth factor 2 and its receptor expressions on corneal repair. Int J Clin Exp Pathol 2015;8:10185–10191. [PMC free article] [PubMed] [Google Scholar]
- 27. Ho TC, Chen SL, Wu JY et al. PEDF promotes self‐renewal of limbal stem cell and accelerates corneal epithelial wound healing. Stem Cells 2013;31:1775–1784. [DOI] [PubMed] [Google Scholar]
- 28. Zhou Q, Chen P, Di G et al. Ciliary neurotrophic factor promotes the activation of corneal epithelial stem/progenitor cells and accelerates corneal epithelial wound healing. Stem Cells 2015;33:1566–1576. [DOI] [PubMed] [Google Scholar]
- 29. Chen J, Chen P, Backman LJ et al. Ciliary neurotrophic factor promotes the migration of corneal epithelial stem/progenitor cells by up‐regulation of MMPs through the phosphorylation of Akt. Sci Rep 2016;6:25870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sun CC, Chiu HT, Lin YF et al. Y‐27632, a ROCK inhibitor, promoted limbal epithelial cell proliferation and corneal wound healing. PLoS One 2015;10:e0144571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ji H, Tang H, Lin H et al. Rho/Rock cross‐talks with transforming growth factor‐β/Smad pathway participates in lung fibroblast‐myofibroblast differentiation. Biomed Rep 2014;2:787–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Yu FS, Yin J, Xu K et al. Growth factors and corneal epithelial wound healing. Brain Res Bull 2010;81:229–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ichikawa H, Nakata N, Abo Y et al. Gene pathway analysis of the mechanism by which the Rho‐associated kinase inhibitor Y‐27632 inhibits apoptosis in isolated thawed human embryonic stem cells. Cryobiology 2012;64:12–22. [DOI] [PubMed] [Google Scholar]
- 34. Saghizadeh M, Soleymani S, Harounian A et al. Alterations of epithelial stem cell marker patterns in human diabetic corneas and effects of c‐met gene therapy. Mol Vis 2011;17:2177–2190. [PMC free article] [PubMed] [Google Scholar]
- 35. Ljubimov AV. Diabetic complications in the cornea. Vision Res 2017; Apr 27. pii: S0042–6989(17)30047–0. doi: 10.1016/j.visres.2017.03.002. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kramerov AA, Saghizadeh M, Ljubimov AV. Adenoviral gene therapy for diabetic keratopathy: Effects on wound healing and stem cell marker expression in human organ‐cultured corneas and limbal epithelial cells. J Vis Exp 2016;7:e54058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Rama P, Matuska S, Paganoni G et al. Limbal stem‐cell therapy and longterm corneal regeneration. N Engl J Med 2010;363:147–155. [DOI] [PubMed] [Google Scholar]
- 38. Rama P, Ferrari G, Pellegrini G. Cultivated limbal epithelial transplantation. Curr Opin Ophthalmol 2017;28:387–389. [DOI] [PubMed] [Google Scholar]
- 39. Erbani J, Aberdam D, Larghero J et al. Pluripotent stem cells and other innovative strategies for the treatment of ocular surface diseases. Stem Cell Rev 2016;12:171–178. [DOI] [PubMed] [Google Scholar]
- 40. Cheng J, Zhai H, Wang J et al. Long‐term outcome of allogeneic cultivated limbal epithelial transplantation for symblepharon caused by severe ocular burns. BMC Ophthalmol 2017;17:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lang SJ, Böhringer D, Geerling G et al. Long‐term results of allogenic penetrating limbo‐keratoplasty: 20 years of experience. Eye (Lond) 2017;31:372–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ramachandran C, Basu S, Sangwan VS et al. Concise review: The coming of age of stem cell treatment for corneal surface damage. Stem Cells Translational Medicine 2014;3:1160–1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Zhao Y, Ma L. Systematic review and meta‐analysis on transplantation of ex vivo cultivated limbal epithelial stem cell on amniotic membrane in limbal stem cell deficiency. Cornea 2015; 34:592–600. [DOI] [PubMed] [Google Scholar]
- 44. Sheth‐Shah R, Vernon AJ, Seetharaman S et al. Regulatory requirements in the good manufacturing practice production of an epithelial cell graft for ocular surface reconstruction. Regen Med 2016;11:307–320. [DOI] [PubMed] [Google Scholar]
- 45. Basu S, Sureka SP, Shanbhag SS et al. Simple limbal epithelial transplantation: Long‐term clinical outcomes in 125 cases of unilateral chronic ocular surface burns. Ophthalmology 2016;123:1000–1010. [DOI] [PubMed] [Google Scholar]
- 46. Pellegrini G, Lambiase A, Macaluso C et al. From discovery to approval of an advanced therapy medicinal product‐containing stem cells in the EU. Regen Med 2016;11:407–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Arora R, Dokania P, Manudhane A et al. Preliminary results from the comparison of simple limbal epithelial transplantation with conjunctival limbal autologous transplantation in severe unilateral chronic ocular burns. Indian J Ophthalmol 2017;65:35–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Vasania VS, Prasad P, Gill RK et al. Molecular and cellular characterization of expanded and cryopreserved human limbal epithelial stem cells reveal unique immunological properties. Exp Eye Res 2011;92:47–56. [DOI] [PubMed] [Google Scholar]
- 49. Pathak M, Cholidis S, Haug K et al. Clinical transplantation of ex vivo expanded autologous limbal epithelial cells using a culture medium with human serum as single supplement: A retrospective case series. Acta Ophthalmol 2013;91:769–775. [DOI] [PubMed] [Google Scholar]
- 50. Menzel‐Severing J, Kruse FE Schlötzer‐Schrehardt U. Stem cell‐based therapy for corneal epithelial reconstruction: Present and future. Can J Ophthalmol 2013;48:13–21. [DOI] [PubMed] [Google Scholar]
- 51. Bobba S, Chow S, Watson S et al. Clinical outcomes of xeno‐free expansion and transplantation of autologous ocular surface epithelial stem cells via contact lens delivery: A prospective case series. Stem Cell Res Ther 2015;6:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Behaegel J, Ní Dhubhghaill S, Koppen C et al. Safety of cultivated limbal epithelial stem cell transplantation for human corneal regeneration. Stem Cells Int 2017;2017:6978253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Massie I, Kureshi AK, Schrader S et al. Optimization of optical and mechanical properties of real architecture for 3‐dimensional tissue equivalents: Towards treatment of limbal epithelial stem cell deficiency. Acta Biomater 2015;24:241–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Sabater AL, Perez VL. Amniotic membrane use for management of corneal limbal stem cell deficiency. Curr Opin Ophthalmol 2017;28:363–369. [DOI] [PubMed] [Google Scholar]
- 55. Saghizadeh M, Winkler MA, Kramerov AA et al. A simple alkaline method for decellularizing human amniotic membrane for cell culture. PLoS One 2013;8:e79632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Yazdanpanah G, Jabbehdari S, Djalilian AR. Limbal and corneal epithelial homeostasis. Curr Opin Ophthalmol 2017;28:348–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Malhotra C, Jain AK. Human amniotic membrane transplantation: Different modalities of its use in ophthalmology. World J Transplant 2014;4:111–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Sheth R, Neale MH, Shortt AJ et al. Culture and characterization of oral mucosal epithelial cells on a fibrin gel for ocular surface reconstruction. Curr Eye Res 2015;40:1077–1087. [DOI] [PubMed] [Google Scholar]
- 59. Yeung AM, Faraj LA, McIntosh OD et al. Fibrin glue inhibits migration of ocular surface epithelial cells. Eye (Lond) 2016;30:1389–1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Nishida K, Yamato M, Hayashida Y et al. Corneal reconstruction with tissue‐engineered cell sheets composed of autologous oral mucosal epithelium. N Engl J Med 2004;351:1187–1196. [DOI] [PubMed] [Google Scholar]
- 61. Chen P, Zhou Q, Wang J et al. Characterization of the corneal surface in limbal stem cell deficiency and after transplantation of cultured allogeneic limbal epithelial cells. Graefes Arch Clin Exp Ophthalmol 2016;254:1765–1777. [DOI] [PubMed] [Google Scholar]
- 62. Eslani M, Haq Z, Movahedan A et al. Late acute rejection after allograft limbal stem cell transplantation: Evidence for long‐term donor survival. Cornea 2017;36:26–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Basu S, Ali H, Sangwan VS. Clinical outcomes of repeat autologous cultivated limbal epithelial transplantation for ocular surface burns. Am J Ophthalmol 2012;153:643–650. 650.e1–2. [DOI] [PubMed] [Google Scholar]
- 64. Tseng SC, Chen SY, Shen YC et al. Critical appraisal of ex vivo expansion of human limbal epithelial stem cells. Curr Mol Med 2010;10:841–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Utheim TP. Concise review: Transplantation of cultured oral mucosal epithelial cells for treating limbal stem cell deficiency‐current status and future perspectives. Stem Cells 2015;33:1685–1695. [DOI] [PubMed] [Google Scholar]
- 66. Kolli S, Ahmad S, Mudhar HS et al. Successful application of ex vivo expanded human autologous oral mucosal epithelium for the treatment of total bilateral limbal stem cell deficiency. Stem Cells 2014;32:2135–2146. [DOI] [PubMed] [Google Scholar]
- 67. Holan V, Trosan P, Cejka C et al. A comparative study of the therapeutic potential of mesenchymal stem cells and limbal epithelial stem cells for ocular surface reconstruction. Stem Cells Transl Med 2015;4:1052–1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Casaroli‐Marano RP, Nieto‐Nicolau N, Martínez‐Conesa EM et al. Potential role of induced pluripotent stem cells (IPSCs) for cell‐based therapy of the ocular surface. J Clin Med 2015;4:318–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Hayashi R, Ishikawa Y, Ito M et al. Generation of corneal epithelial cells from induced pluripotent stem cells derived from human dermal fibroblast and corneal limbal epithelium. PLoS One 2012;7:e45435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Sareen D, Saghizadeh M, Ornelas L et al. Differentiation of human limbal‐derived induced pluripotent stem cells into limbal‐like epithelium. Stem Cells Transl Med 2014;3:1002–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Mikhailova A, Ilmarinen T, Ratnayake A et al. Human pluripotent stem cell‐derived limbal epithelial stem cells on bioengineered matrices for corneal reconstruction. Exp Eye Res 2016;146:26–34. [DOI] [PubMed] [Google Scholar]
- 72. Funderburgh JL, Funderburgh ML, Du Y. Stem cells in the limbal stroma. Ocul Surf 2016;14:113–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Branch MJ, Hashmani K, Dhillon P et al. Mesenchymal stem cells in the human corneal limbal stroma. Invest Ophthalmol Vis Sci 2012;53:5109–5116. [DOI] [PubMed] [Google Scholar]
- 74. Veréb Z, Póliska S, Albert R et al. Role of human corneal stroma‐derived mesenchymal‐like stem cells in corneal immunity and wound healing. Sci Rep 2016;6:26227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Thill M, Schlagner K, Altenähr S et al. A novel population of repair cells identified in the stroma of the human cornea. Stem Cells Dev 2007;16:733–745. [DOI] [PubMed] [Google Scholar]
- 76. Basu S, Hertsenberg AJ, Funderburgh ML et al. Human limbal biopsy‐derived stromal stem cells prevent corneal scarring. Sci Transl Med 2014;6:266ra172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Hertsenberg AJ, Shojaati G, Funderburgh ML et al. Corneal stromal stem cells reduce corneal scarring by mediating neutrophil infiltration after wounding. PLoS One 2017;12:e0171712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Morgan SR, Dooley EP, Kamma‐Lorger C et al. Early wound healing of laser in situ keratomileusis‐like flaps after treatment with human corneal stromal stem cells. J Cataract Refract Surg 2016;42:302–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Almaliotis D, Koliakos G, Papakonstantinou E et al. Mesenchymal stem cells improve healing of the cornea after alkali injury. Graefes Arch Clin Exp Ophthalmol 2015;253:1121–1135. [DOI] [PubMed] [Google Scholar]
- 80. Demirayak B, Yüksel N, Çelik OS et al. Effect of bone marrow and adipose tissue‐derived mesenchymal stem cells on the natural course of corneal scarring after penetrating injury. Exp Eye Res 2016;151:227–235. [DOI] [PubMed] [Google Scholar]
- 81. Naylor RW, McGhee CN, Cowan CA et al. Derivation of corneal keratocyte‐like cells from human induced pluripotent stem cells. PLoS One 2016;11:e0165464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Joyce NC, Harris DL, Mello DM. Mechanisms of mitotic inhibition in corneal endothelium: Contact inhibition and TGF‐β2. Invest Ophthalmol Vis Sci 2002;43:2152–2159. [PubMed] [Google Scholar]
- 83. Mimura T, Yamagami S, Amano S. Corneal endothelial regeneration and tissue engineering. Prog Retin Eye Res 2013;35:1–17. [DOI] [PubMed] [Google Scholar]
- 84. de Araujo AL, Gomes JÁ. Corneal stem cells and tissue engineering: Current advances and future perspectives. World J Stem Cells 2015;7:806–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Bartakova A, Kunzevitzky NJ, Goldberg JL. Regenerative cell therapy for corneal endothelium. Curr Ophthalmol Rep 2014;2:81–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Joyce NC, Harris DL, Markov V et al. Potential of human umbilical cord blood mesenchymal stem cells to heal damaged corneal endothelium. Mol Vis 2012;18:547e564. [PMC free article] [PubMed] [Google Scholar]
- 87. Ju C, Zhang K, Wu X. Derivation of corneal endothelial cell‐like cells from rat neural crest cells in vitro. PLoS One 2012;e42378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Meekins LC, Rosado‐Adames N, Maddala R et al. Corneal endothelial cell migration and proliferation enhanced by Rho kinase (ROCK) inhibitors in in vitro and in vivo models. Invest Ophthalmol Vis Sci 2016;57:6731–6738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Chen P, Chen JZ, Shao CY et al. Treatment with retinoic acid and lens epithelial cell‐conditioned medium in vitro directed the differentiation of pluripotent stem cells towards corneal endothelial cell‐like cells. Exper Ther Med 2015;9:351–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Zhao JJ, Afshari NA. Generation of human corneal endothelial cells via in vitro ocular lineage restriction of pluripotent stem cells. Invest Ophthalmol Vis Sci 2016;57:6878–6884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Zhang K, Pang K, Wu X. Isolation and transplantation of corneal endothelial cell‐like cells derived from in‐vitro‐differentiated human embryonic stem cells. Stem Cells Dev 2014;23:1340–1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Song Q, Yuan S, An Q et al. Directed differentiation of human embryonic stem cells to corneal endothelial cell‐like cells: A transcriptomic analysis. Exp Eye Res 2016;151:107–114. [DOI] [PubMed] [Google Scholar]
- 93. McCabe KL, Kunzevitzky NJ, Chiswell BP et al. Efficient generation of human embryonic stem cell‐derived corneal endothelial cells by directed differentiation. PLoS One 2015;10:e0145266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Abrahimi P, Qin L, Chang WG et al. Blocking MHC class II on human endothelium mitigates acute rejection. JCI Insight 2016;1:e85293. [DOI] [PMC free article] [PubMed] [Google Scholar]


