Figure 1.
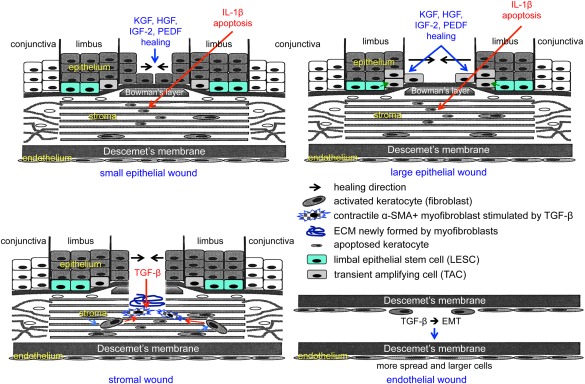
Schematic representation of main events during corneal epithelial, stromal, and endothelial wound healing. Top left, healing of small epithelial wound under the influence of several growth factors entails participation of central cells only. Keratocytes under the wound die by apoptosis mediated by epithelium‐derived interleukin‐1β. Top right, healing of large epithelial wound under the influence of several growth factors entails participation of both limbal epithelial stem cells and their progeny (transient amplifying cells), as well as of central cells. Bottom left, healing of a stromal wound entails activation of keratocytes to form fibroblasts that are transformed to motile myofibroblasts under the influence of transforming growth factor (TGF)‐β. Myofibroblasts positive for α‐smooth muscle actin contract the wound, and also produce and remodel the extracellular matrix in the wound bed. Burns are also associated with stromal neovascularization (not shown). Bottom right, healing of endothelial wound entails epithelial–mesenchymal transformation (EMT) and cell migration under the influence of TGF‐β. Wound closure is accompanied by increased spreading and enlargement of endothelial cells that undergo the process opposite to EMT, that is, mesenchymal–epithelial transformation. Abbreviations: ECM, extracellular matrix; EMT, epithelial–mesenchymal transformation; HGF, hepatocyte growth factor; IGF‐2, insulin‐like growth factor‐2; IL, interleukin; KGF, keratinocyte growth factor; PEDF, pigment epithelium‐derived factor; TGF, transforming growth factor; α‐SMA, α‐smooth muscle actin.
