Abstract
Aims
To assess the impact of baseline characteristics on clinical outcomes in the LixiLan‐L trial, a randomized open‐label trial designed to evaluate the efficacy and safety of iGlarLixi, a novel fixed‐ratio combination of insulin glargine 100 U (iGlar) plus lixisenatide, in comparison with iGlar over 30 weeks in a population of patients with type 2 diabetes mellitus (T2DM) inadequately controlled on a previous regimen of basal insulin alone or in combination with 1 or 2 oral glucose‐lowering drugs.
Materials and Methods
In this exploratory analysis of LixiLan‐L (N = 736), efficacy outcomes were assessed within population subgroups derived from the following baseline characteristics: glycated haemoglobin [HbA1c; <8%, ≥8% (<64, ≥64 mmol/mol)]; duration of T2DM (<10, ≥10 years); body mass index (<30, ≥30 kg/m2). Furthermore, the incidence of symptomatic hypoglycaemia with plasma glucose ≤3.9 mmol/L (≤70 mg/dL) was also analysed according to the same subgroups.
Results
Compared with the iGlar treatment group, patients treated with iGlarLixi showed consistently greater reductions in HbA1c during the treatment period, with higher percentages of patients achieving the HbA1c target level of <7% (<53 mmol/mol) in all of the subpopulations tested (P < .0001 for all), having consistent mitigation of body weight gain and with no major differences in the incidence of hypoglycaemia.
Conclusions
iGlarLixi consistently improved glycaemic control compared with iGlar in all baseline characteristic subgroups of patients with T2DM inadequately controlled with insulin, including difficult‐to‐treat subgroups of patients with long duration of diabetes, obesity and high HbA1c.
Clinical trial number: NCT02058160 (clinicaltrials.gov).
Keywords: GLP‐1, glycaemic control, insulin therapy, type 2 diabetes
1. INTRODUCTION
Current diabetes treatment guidelines suggest that if a patient with type 2 diabetes mellitus (T2DM) does not achieve glycated haemoglobin (HbA1c) targets despite treatment with basal insulin (usually in combination with metformin and/or another oral drug), the patient's regimen should be intensified with the addition of either mealtime insulin or a glucagon‐like peptide‐1 receptor agonist (GLP‐1 RA).1, 2 The addition of mealtime insulin to a basal regimen, while often effective at lowering glycaemia, can involve markedly increasing the patient's daily injection burden, and may be accompanied by an increased risk of weight gain and hypoglycaemia. Adding a GLP‐1 RA to a basal insulin regimen can augment glycaemic control with minimal additional risk of hypoglycaemia and without adding to the risk of weight gain associated with insulin itself, although GLP‐1 RAs are often associated with gastrointestinal adverse events. Lixisenatide (Lixi: Lyxumia, Sanofi, Paris, France; Adlyxin, Sanofi, Bridgewater, NJ, USA) is a once‐daily prandial GLP‐1 RA with a robust postprandial plasma glucose (PPG)‐lowering effect, which is predominantly driven by delayed gastric emptying and reductions in glucose‐dependent glucagon release.3, 4, 5, 6 The rationale for combining basal insulin with Lixi is that glycaemic control can be enhanced via a dual, complementary mechanism of action offered by the two components, each of which has its own distinct mechanism: basal insulin principally lowers the patient's fasting plasma glucose (FPG), while Lixi acts predominantly by lowering PPG.
The combination of Lixi and basal insulin glargine 100 U (iGlar) as separate injections has been shown to be more effective than basal insulin alone.7, 8 iGlarLixi, a titratable fixed‐ratio combination of iGlar and Lixi, was developed for administration as a single daily injection, and therefore has the potential to increase glycaemic control without the need for additional injections.
The LixiLan‐L trial showed that iGlarLixi achieved superior glycaemic control compared with iGlar in patients with advanced T2DM whose glycaemia was not adequately controlled on basal insulin in combination with up to two oral glucose‐lowering drugs. iGlarLixi mitigated the weight gain observed with iGlar, did not have additional risk of hypoglycaemia compared with iGlar alone, and had a lower rate of gastrointestinal side‐effects compared with data from prior studies of Lixi.9 Similarly, in the LixiLan‐O trial, iGlarLixi was superior to iGlar and to Lixi in insulin‐naïve patients with T2DM inadequately controlled with metformin ± a second oral glucose‐lowering drug.10 Trials of another single injection combination of a GLP‐1 RA with basal insulin, in similar populations to that of the present study, also found additional benefit of the combination over basal insulin alone.11, 12
As patients with T2DM exhibit diverse disease characteristics, it is important to determine the efficacy and safety of new treatment options across the patient spectrum.13, 14, 15 Therefore, exploratory subpopulation analyses of data from the LixiLan‐L trial were performed to assess the impact of patients’ baseline characteristics of HbA1c, disease duration and body mass index (BMI) on clinical outcomes.
2. MATERIALS AND METHODS
2.1. Trial design
The full methodology of the LixiLan‐L trial (NCT02058160) has been described previously in detail.9 Briefly, LixiLan‐L was an open‐label, randomized, parallel‐group, multinational, multicentre, 2‐arm trial with treatment duration of 30 weeks. The trial was designed and monitored in accordance with Good Clinical Practice, the International Conference on Harmonization, and the Declaration of Helsinki. Institutional review boards or ethics committees at each study site approved the protocol. Each patient gave written informed consent.
2.2. Trial population
Patients (≥18 years) with T2DM diagnosed at least 1 year prior to screening were eligible for inclusion if they had been treated with basal insulin for at least 6 months before screening and were under a stable regimen of 15 to 40 U/d (±20%) for at least 2 months prior to screening. The permitted oral glucose‐lowering therapies (a maximum of two was allowed) were metformin (>1500 mg/d or maximum‐tolerated dose), a sulphonylurea, a glinide, a sodium‐glucose cotransporter 2 inhibitor or a dipeptidyl peptidase 4 inhibitor. Doses of any oral glucose‐lowering therapies (if taken) also had to have been stable during the 3 months before screening.
2.3. Randomization and interventions
Eligible patients entered a 6‐week run‐in phase during which any oral glucose‐lowering drug other than metformin was stopped, patients were switched to iGlar (if they had previously been receiving another basal insulin), and the daily dose of iGlar was titrated and/or stabilized for all patients. To enter the subsequent treatment period, patients were required to have an HbA1c level of 7% to 10% (53 to 86 mmol/mol), mean fasting self‐measured plasma glucose ≤7.8 mmol/L (≤140 mg/dL), iGlar daily dose 20 to 50 U (inclusive), calcitonin ≤5.9 pmol/L, and amylase and/or lipase levels <3× the upper limit of normal at the end of the run‐in period. Patients who met these requirements were then randomized in a 1:1 ratio to receive once‐daily open‐label treatment with either iGlarLixi or iGlar for 30 weeks. Titration algorithms and criteria for rescue medication have been described previously.9 Briefly, both treatments were titrated to FPG <100 mg/dL (<5.6 mmol/mol) up to a maximum dose of 60 U of insulin/day. iGlarLixi was administered via two pen injector options: pen A delivered a ratio of 2 U iGlar to 1 µg Lixi over a 10 to 40 U iGlar dose range; pen B delivered a ratio of 3 U iGlar to 1 µg Lixi over a 30 to 60 U iGlar dose range. The dose of iGlarLixi was titrated as per the patient's insulin requirement, and the accompanying Lixi dose was driven by the applied ratio, with a maximum dose of 20 µg/d.
2.4. Subpopulation analysis
The exploratory subpopulation analysis presented here was designed to evaluate the potential impact of baseline disease characteristics on the clinical outcomes of the LixiLan‐L trial; participants were split into subpopulations according to the following baseline characteristics: randomization strata of HbA1c at week −1 in the trial protocol [<8% or ≥8% (<64 or ≥64 mmol/mol); prespecified cut‐offs in the trial population, with 64 mmol/mol also being the approximate baseline mean for the study population]; duration of T2DM [<10 or ≥10 years: the approximate median duration for the trial population (categories determined post hoc)]; and BMI (<30 or ≥30 kg/m2; prespecified cut‐offs in the trial protocol). Clinical endpoints, from baseline to week 30, were analysed post hoc by the above subpopulation categories: change in HbA1c from baseline to week 30 (prespecified for HbA1c and BMI categories); FPG; 2‐h PPG levels during a standardized breakfast meal test; body weight; and the proportion of patients who achieved HbA1c <7% (<53 mmol/mol) were also recorded for each subpopulation category. Documented symptomatic hypoglycaemia, defined as an event with typical symptoms of hypoglycaemia that were accompanied by measured plasma glucose concentration of ≤3.9 mmol/L (≤70 mg/dL), was also analysed per subpopulation.
2.5. Statistical methods
A 2‐way analysis of variance with last observation carried forward was used to assess comparisons between treatment groups and subpopulations for continuous data. Comparisons between treatment groups and subpopulations for categorical data were based on Cochran–Mantel–Haenszel tests. Analysis of covariance was carried out in accordance with prior publications on this study.9 Heterogeneity was tested using the 1 degree of freedom contrast corresponding to the test of treatment by subgroup interaction.
3. RESULTS
3.1. Main study analysis
A total of 736 patients were randomized in the LixiLan‐L trial: 367 to iGlarLixi and 369 to iGlar. Demographics and baseline characteristics were similar across the two treatment groups, and have been published previously with the primary study results.9 In the main study analysis, the least squares mean HbA1c change from baseline was significantly greater with iGlarLixi [−1.1% (−12 mmol/mol)] than with iGlar [−0.6% (−6.6 mmol/mol)]. In total, 55% of iGlarLixi patients reached HbA1c <7% (<53 mmol/mol) compared with 30% of iGlar patients.9 iGlarLixi also demonstrated significant reductions in 2‐h PPG following a standardized liquid breakfast meal compared with iGlar. Mean body weight decreased by 0.7 kg with iGlarLixi and increased by 0.7 kg with iGlar (P < .0001). These improvements were accompanied by no increased risk of hypoglycaemia with iGlarLixi compared with iGlar.
Similar numbers of patients from the iGlarLixi and iGlar treatment groups fell into each of the subgroups based on baseline HbA1c, diabetes duration and BMI, allowing for balanced subgroup analyses.
3.2. Glycaemic control according to baseline HbA1c, duration of T2DM and BMI
Table 1 shows efficacy analyses in subpopulations based on baseline HbA1c [<8% or ≥8% (<64 or ≥64 mmol/mol), duration of T2DM (<10 or ≥10 years) and BMI (<30 or ≥30 kg/m2)]. In all subpopulations, reductions from baseline to week 30 in HbA1c were consistently greater with iGlarLixi than with iGlar (P < .0001). Within both the iGlarLixi and the iGlar treatment groups, patients with baseline HbA1c ≥8% (≥64 mmol/mol) achieved significantly greater HbA1c reductions than those with baseline HbA1c <8% (<64 mmol/mol; P < .001). In each of the subpopulations based on baseline HbA1c, T2DM duration and BMI, the proportion of patients who achieved target HbA1c <7% (<53 mmol/mol) was higher with iGlarLixi than with iGlar alone (P < .0001; Figure 1A–C).
Table 1.
Subpopulation analyses according to baseline HbA1c, duration of T2DM and BMI
| Subpopulation analyses according to baseline HbA1c | |||||
|---|---|---|---|---|---|
| iGlarLixi (fixed‐ratio combination) | iGlar (insulin glargine 100 U) | ||||
| HbA1c <8% | HbA1c ≥8% | HbA1c <8% | HbA1c ≥8% | ||
| HbA1c (%) | Baseline (n) | 7.5 ± 0.3 (165) | 8.6 ± 0.5 (199) | 7.4 ± 0.4 (163) | 8.6 ± 0.5 (201) |
| Week 30 | 6.7 ± 0.8 | 7.2 ± 0.9 | 7.1 ± 0.8 | 7.8 ± 0.9 | |
| Mean change at week 30 | −0.8 ± 0.8 | −1.4 ± 0.9a | −0.3 ± 0.8 | −0.8 ± 0.9a | |
| Mean difference ± SEb | −0.5 ± 0.1 | −0.5 ± 0.1 | |||
| P‐value | <.0001 | <.0001 | |||
| FPG (mmol/L) | Baseline (n) | 7.3 ± 1.9 (165) | 7.3 ± 2.0 (199) | 6.9 ± 1.9 (163) | 7.6 ± 2.2 (201) |
| Mean change at week 30 | −0.6 ± 2.6 | −0.4 ± 2.7 | −0.3 ± 2.7 | −0.8 ± 2.7 | |
| Mean difference ± SEb | −0.3 ± 0.3 | 0.5 ± 0.3 | |||
| P‐value | .370 | .080 | |||
| 2‐h PPG (mmol/L) | Baseline (n) | 13.9 ± 3.4 (152) | 15.7 ± 4.0 (180) | 13.7 ± 3.2 (151) | 16.0 ± 3.7 (189) |
| Mean change at week 30 | −4.6 ± 3.9 | −5.3 ± 4.9a | −0.8 ± 4.2 | −2.2 ± 4.2a | |
| Mean difference ± SEb | −3.8 ± 0.5 | −3.1 ± 0.5 | |||
| P‐value | <.0001 | <.0001 | |||
| Daily iGlar dose at week 30 (U) | n | 165 | 200 | 163 | 202 |
| Mean ± SD | 45.6 ± 12.1 | 45.8 ± 13.5 | 44.8 ± 13.2 | 48.1 ± 11.8 | |
| Subpopulation analyses according to T2DM duration | |||||
|---|---|---|---|---|---|
| iGlarLixi (fixed‐ratio combination) | iGlar (insulin glargine 100 U) | ||||
| <10 years | ≥10 years | <10 years | ≥10 years | ||
| T2DM duration (years) | Baseline (n) | 6.7 ± 2.1 (167) | 16.6 ± 5.7 (199) | 6.1 ± 2.5 (150) | 16.3 ± 5.7 (214) |
| HbA1c (%) | Baseline (n) | 8.0 ± 0.7 (166) | 8.1 ± 0.7 (198) | 8.0 ± 0.7 (150) | 8.1 ± 0.7 (213) |
| Week 30 | 6.9 ± 0.9 | 7.0 ± 0.9 | 7.4 ± 1.0 | 7.6 ± 0.9 | |
| Mean change at week 30 | −1.1 ± 0.9 | −1.1 ± 0.9 | −0.6 ± 0.9 | −0.6 ± 0.8 | |
| Mean difference ± SEb | −0.5 ± 0.1 | −0.5 ± 0.1 | |||
| P‐value | <.0001 | <.0001 | |||
| FPG (mmol/L) | Baseline (n) | 7.5 ± 2.0 (166) | 7.2 ± 1.9 (198) | 7.3 ± 1.9 (150) | 7.3 ± 2.2 (213) |
| Mean change at week 30 | −0.9 ± 2.5 | −0.1 ± 2.8a | −0.6 ± 2.5 | −0.6 ± 2.8 | |
| Mean difference ± SEb | −0.3 ± 0.3 | 0.5 ± 0.3 | |||
| P‐value | .352 | .067 | |||
| 2‐h PPG (mmol/L) | Baseline (n) | 14.6 ± 3.8 (151) | 15.1 ± 3.9 (181) | 15.0 ± 3.4 (138) | 15.0 ± 3.9 (201) |
| Mean change at week 30 | −4.8 ± 4.6 | −5.1 ± 4.4 | −1.8 ± 3.8 | −1.4 ± 4.5 | |
| Mean difference ± SEb | −3.0 ± 0.5 | −3.6 ± 0.4 | |||
| P‐value | <.0001 | <.0001 | |||
| Daily iGlar dose at week 30 (U) | n | 166 | 199 | 150 | 214 |
| Mean ± SD | 46.4 ± 12.8 | 45.1 ± 12.9 | 48.6 ± 12.4 | 45.2 ± 12.4 | |
| Subpopulation analyses according to BMI | |||||
|---|---|---|---|---|---|
| iGlarLixi (fixed‐ratio combination) | iGlar (insulin glargine 100 U) | ||||
| BMI <30 kg/m2 | BMI ≥30 kg/m2 | BMI <30 kg/m2 | BMI ≥30 kg/m2 | ||
| BMI (kg/m2) | Baseline (n) | 27.3 ± 1.9 (156) | 34.3 ± 2.8 (210) | 27.2 ± 2.0 (156) | 33.8 ± 2.8 (209) |
| HbA1c (%) | Baseline (n) | 8.1 ± 0.7 (155) | 8.1 ± 0.7 (209) | 8.1 ± 0.8 (156) | 8.1 ± 0.7 (208) |
| Week 30 | 7.0 ± 0.9 | 7.0 ± 0.9 | 7.6 ± 1.0 | 7.4 ± 0.8 | |
| Mean change at week 30 | −1.1 ± 0.9 | −1.1 ± 0.9 | −0.5 ± 0.9 | −0.6 ± 0.9 | |
| Mean difference ± SEb | −0.6 ± 0.1 | −0.5 ± 0.1 | |||
| P‐value | <.0001 | <.0001 | |||
| FPG (mmol/L) | Baseline (n) | 7.0 ± 2.0 (155) | 7.6 ± 1.8 (209) | 6.9 ± 2.2 (156) | 7.6 ± 1.9 (208) |
| Mean change at week 30 | −0.2 ± 2.8 | −0.6 ± 2.6 | −0.3 ± 3.0 | −0.8 ± 2.4 | |
| Mean difference ± SEb | 0.1 ± 0.3 | 0.2 ± 0.3 | |||
| P‐value | .739 | .516 | |||
| 2‐h PPG (mmol/L) | Baseline (n) | 14.7 ± 3.7 (135) | 15.0 ± 3.9 (197) | 14.9 ± 3.9 (143) | 15.0 ± 3.5 (197) |
| Mean change at week 30 | −5.0 ± 4.7 | −4.9 ± 4.3 | −0.8 ± 4.5 | −2.1 ± 3.9a | |
| Mean difference ± SEb | −4.2 ± 0.5 | −2.8 ± 0.4c | |||
| P‐value | <.0001 | <.0001 | |||
| Daily iGlar dose at week 30 (U) | n | 155 | 210 | 156 | 209 |
| Mean ± SD | 40.6 ± 12.4 | 49.5 ± 11.9 | 42.8 ± 12.7 | 49.5 ± 11.6 | |
Abbreviations: BMI, body mass index; FPG, fasting plasma glucose; HbA1c, glycated haemoglobin; iGlar, insulin glargine; Lixi, lixisenatide; PPG, postprandial plasma glucose; SE, standard error; T2DM, type 2 diabetes mellitus.
All data are mean ± SD unless stated otherwise.
Between‐subpopulation comparison P < .05.
Mean difference iGlarLixi vs iGlar. Treatment comparison P‐values based on 2‐factor analysis of variance.
Heterogeneity P‐value = .012. Value is based on a single degree of freedom contrast of treatment differences between subgroups.
Figure 1.
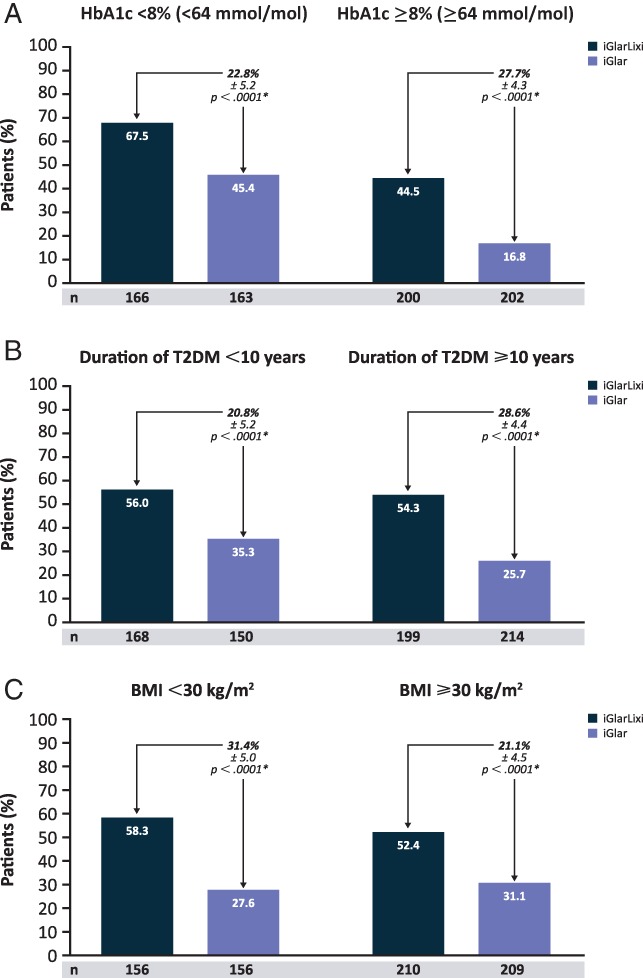
Percentages of patients who achieved HbA1c target <7% (<53 mmol/mol) for baseline A, HbA1c; B, diabetes duration and C, BMI subpopulations. *Treatment comparisons within subgroups are based on 2‐factor Cochran–Mantel–Haenszel. An adjustment was made for the treatment differences based on the randomization strata. BMI, body mass index; HbA1c, glycated haemoglobin; T2DM, type 2 diabetes mellitus
Similar to the primary LixiLan‐L analyses, there were no significant differences between the 2 treatment groups in mean change in FPG from baseline to week 30 for each subpopulation, indicating the consistent titration of the basal insulin components in the study. However, also in line with the primary results, significant reductions from baseline to week 30 in 2‐h PPG during the standardized meal test were observed with iGlarLixi vs iGlar across subpopulations (P < .0001). Within both the iGlarLixi and the iGlar treatment groups, patients with baseline HbA1c ≥8% (≥64 mmol/mol) achieved significantly greater reductions in 2‐h PPG than those with baseline HbA1c <8% (<64 mmol/mol; P < .001).
3.3. Body weight change by subpopulation
Consistent with the primary LixiLan‐L results, iGlarLixi mitigated body weight gain compared with iGlar alone across all baseline characteristic subpopulations (P ≤ .001; Figure 2A–C). Irrespective of baseline HbA1c, T2DM duration or BMI, iGlarLixi treatment resulted in mean body weight reduction (up to −0.9 kg), whereas iGlar treatment resulted in body weight gain (up to +1.1 kg).
Figure 2.
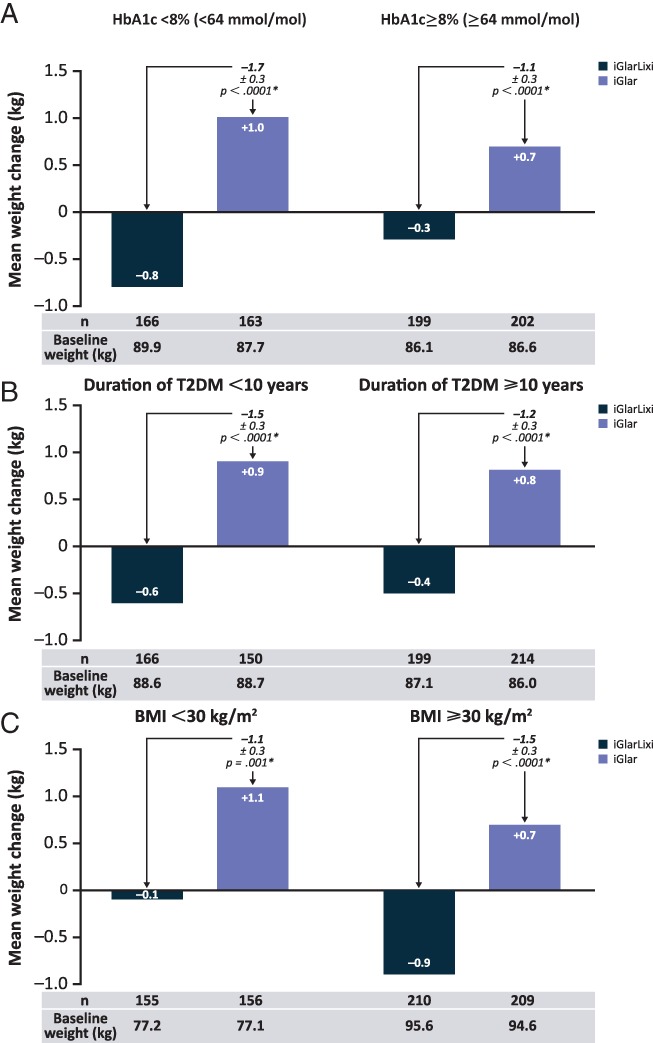
Mean weight change for baseline A, HbA1c; B, diabetes duration and C, BMI subpopulations. *Treatment comparisons within subgroups are based on 2‐factor Cochran–Mantel–Haenszel. BMI, body mass index; HbA1c, glycated haemoglobin; T2DM, type 2 diabetes mellitus
3.4. Hypoglycaemia by subpopulation
The incidence of hypoglycaemia [defined as plasma glucose ≤3.9 mmol/L (≤70 mg/dL)] varied slightly between the HbA1c, T2DM duration and BMI subpopulations; however, no significant differences were observed (Figure 3A–C). The corresponding number of events per patient‐year was numerically lower in the iGlarLixi group than in the iGlar group in each of the subpopulation analyses.
Figure 3.
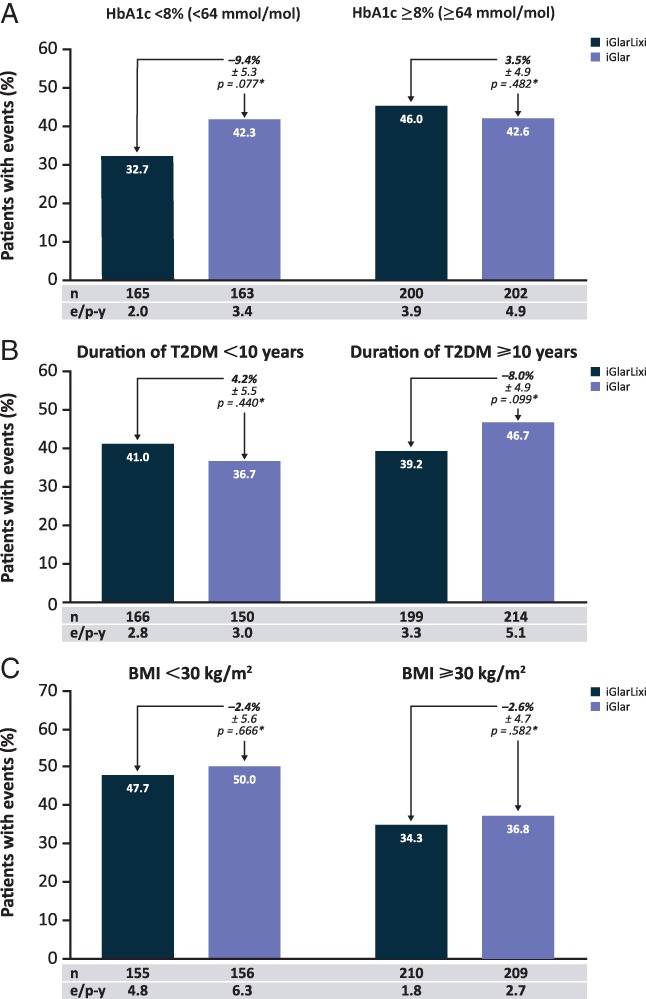
Hypoglycaemia incidence for baseline A, HbA1c; B, diabetes duration and C, BMI subpopulations. *Weighted average of proportion difference between treatment groups. Treatment comparisons are based on 2‐factor Cochran–Mantel–Haenszel. An adjustment was made for the treatment differences based on the randomization strata. Hypoglycaemia defined as plasma glucose ≤3.9 mmol/L (≤70 mg/dL). BMI, body mass index; e/p‐y, events per patient year; HbA1c, glycated haemoglobin; T2DM, type 2 diabetes mellitus
4. DISCUSSION
The subpopulation analyses of the LixiLan‐L trial presented here found that iGlarLixi was effective in all baseline subpopulations tested, including in patients with higher HbA1c, longer duration of diabetes and higher BMI. The iGlarLixi treatment group showed consistently greater glycaemic control and higher percentages of patients reaching the HbA1c target than the iGlar group across all of the subpopulations. The incidence of hypoglycaemia varied between subpopulations, but no substantial differences were observed between therapeutic groups. Additionally, weight gain was mitigated in the iGlarLixi group compared with iGlar, regardless of baseline HbA1c, T2DM duration or BMI. The results of these subpopulation analyses are consistent with those of the main LixiLan‐L trial.9
With a mean diabetes duration of 12 years and mean BMI of 31 kg/m2, the population of the LixiLan‐L trial represents a group of patients with T2DM that is challenging to treat successfully in the clinical setting. These are overweight or obese patients who have long‐standing disease, and have progressed to a stage where they require more than oral glucose‐lowering drugs and injected basal insulin to maintain glycaemic control. By the time of screening for the LixiLan‐L trial, the study patients had a mean HbA1c of 8.5% (69 mmol/mol), despite having been treated for several years with basal insulin and oral drugs.9 In this trial, treatment with iGlar alone reduced the mean HbA1c to 7.5% (58 mmol/mol), which, considering the patients’ history of basal insulin treatment, was a marked improvement and consistent with other trials where basal insulin was titrated.16 However, treatment with iGlarLixi resulted in even further improvements in HbA1c [reduction to 6.9% (52 mmol/mol) for overall population and to ~7.0% (53 mmol/mol) for each subgroup analysed], while also providing both fasting and postprandial glycaemic control with a single injection formulation, which greatly simplifies the therapeutic approach.
The rationale for the combination of iGlar with Lixi is that the two agents have complementary mechanisms of action, with the insulin component mainly acting on the FPG, and the Lixi component reducing PPG levels. Such complementary mechanisms may be particularly beneficial to patients with long‐standing disease and high HbA1c despite basal insulin treatment, especially those with uncontrolled PPG. As observed in the present analysis, iGlarLixi significantly reduced PPG compared with iGlar alone, irrespective of baseline HbA1c, T2DM duration or BMI. This is consistent with the known prandial mechanism of action of Lixi, which has been seen in trials to lower PPG by between 3 and 8 mmol/L (54 and 144 mg/dL).7, 8, 17, 18, 19, 20 This prandial effect, which is an important contributor to the overall glucose‐lowering effect of Lixi, is mediated by a delay in gastric emptying after a meal.6 The delay in gastric emptying is preserved in patients with diabetes, even after the loss of much of their β‐cell function, allowing such patients to maintain a meaningful response to Lixi treatment.
The primary efficacy endpoint was also analysed for other prespecified subgroups (race, ethnicity, age group, sex, metformin use at screening, and number of oral drugs used at screening). Although the data are not presented here, the results of all these analyses were consistent with the overall analysis.
The results of the current exploratory analysis should be interpreted with caution. The limitations of the LixiLan‐L trial and this subsequent post hoc analysis include the open‐label design, the relatively short 30‐week duration, and inclusion criteria that may not completely represent a “real world” population. These limitations may in the future, at least in part, be addressed in studies of longer duration in populations of interest.
In summary, this subgroup analysis of the LixiLan‐L trial acknowledges that, even within a population of patients with long‐standing diabetes, there are variations between patients in terms of disease characteristics. Therefore, while treatment with iGlarLixi was efficacious in the overall LixiLan‐L population, it was important to determine whether benefit would be seen across this spectrum of patient characteristics. The results presented here show that iGlarLixi was beneficial in all of the subgroups studied in the LixiLan‐L trial, regardless of the patients’ demographics and initial clinical characteristics. The glycaemic control was achieved without an increased risk of hypoglycaemia, even in patients with longer duration of disease and low BMI, subgroups that may be considered to be more vulnerable to hypoglycaemic episodes. These findings serve to validate further the concept that the fixed‐ratio titratable combination iGlarLixi is a suitable therapeutic option across a range of patients with differing characteristics who are inadequately controlled on basal insulin treatment.
ACKNOWLEDGEMENTS
The parent study (NCT02058160) of this analysis was funded by Sanofi. Medical writing support was provided by Steve Smith for Caudex (Oxford, UK) and was funded by Sanofi.
Conflict of interest
C. W. has served on advisory boards for AstraZeneca, Janssen and Sanofi; as a consultant for AstraZeneca, Janssen and Sanofi; and received research support from AstraZeneca and Novo Nordisk; participated in speakers’ bureau for AstraZeneca, Boehringer Ingelheim, Eli Lilly, Janssen, Novo Nordisk and Sanofi. R. C. B. has served on advisory panels for Amgen, Eli Lilly and Sanofi; participated in speakers’ bureau for AstraZeneca, Bristol‐Myers Squibb, Eli Lilly, Merck and Sanofi. V. R. A. has served as a consultant for Adocia, Janssen, Novo Nordisk and Sanofi, and is an employee of MedStar Health Research Institute, which has received research support from Amylin, AstraZeneca, Bristol‐Myers Squibb, Boehringer Ingelheim, Eisai, GI Dynamics, GlaxoSmithKline, Halozyme, Hanmi, Intarcia, Janssen, Novo Nordisk, Sanofi and Takeda. M. P. D. has served on advisory panels for Sanofi and Lilly; participated in speakers’ bureau for Sanofi, Lilly, Novo Nordisk, AstraZeneca, Janssen and MSD. C. K. has received research support from Adocia, AstraZeneca, Biocon, Boehringer Ingelheim, Dance Biopharm, Gulf Pharmaceutical Industries, Johnson & Johnson, Eli Lilly, Marvel LifeSciences, Medtronic, Medimmune, Novo Nordisk, Novartis, Roche Diagnostics, Sanofi, Senseonics and Zealand Pharma; and has received advisory, speaker and travel grants from Sanofi. W. S., C. Y., E. N. and E. S. are employees of, and own stock/shareholders in, Sanofi. R. M. B. has served on a scientific advisory board, consulted or performed clinical research with Abbott Diabetes Care, Bayer, Becton Dickinson, Boehringer Ingelheim, AstraZeneca, DexCom, Eli Lilly, Halozyme, Hygieia, Johnson & Johnson, Medtronic, Merck, Novo Nordisk, Roche, Sanofi and Takeda. R. M. B.’s employer, the non‐profit HealthPartners Institute, contracts for his services and no personal income goes to Dr Bergenstal. He has inherited Merck stock.
Author contributions
C. W. participated in data collection, analysis and in writing of the manuscript; and gave final approval of the manuscript. R. C. B. discussed the data and their interpretation, and participated in writing and editing the manuscript; and gave final approval of the manuscript. V. R. A. conducted the clinical trial and collected data, reviewed, edited and revised manuscript drafts; and gave final approval of the manuscript. M. P. D. participated in data analysis, writing the manuscript; and gave final approval of the manuscript. C. K. participated in data analysis, critical review of the manuscript; and gave final approval of the manuscript. W. S. participated in data analysis, critical review of the manuscript; and gave final approval of the manuscript. C. Y. led the data analysis and participated in writing the manuscript; and gave final approval of the manuscript. E. N. undertook main study supervision, analysis and reporting, subpopulation analysis evaluation; and gave final approval of the manuscript. E. S. participated in study design and conduct, data collection and analysis, critical review of the manuscript; and gave final approval of the manuscript. R. M. B. participated in study design and conduct, data collection and analysis, critical review of the manuscript; and gave final approval of the manuscript. All authors confirm that they meet the International Committee of Medical Journal Editors uniform requirements for authorship, and that all authors have read, reviewed and agreed to the final version.
Wysham C, Bonadonna RC, Aroda VR, Puig Domingo M, Kapitza C, Stager W, Yu C, Niemoeller E, Souhami E, Bergenstal RM on behalf of the LixiLan‐L trial investigators . Consistent findings in glycaemic control, body weight and hypoglycaemia with iGlarLixi (insulin glargine/lixisenatide titratable fixed‐ratio combination) vs insulin glargine across baseline HbA1c, BMI and diabetes duration categories in the LixiLan‐L trial. Diabetes Obes Metab. 2017;19:1408–1415. https://doi.org/10.1111/dom.12961
Funding information The parent study (NCT02058160) of this analysis was funded by Sanofi. Medical writing support was provided by Steve Smith for Caudex (Oxford, UK) and was funded by Sanofi.
REFERENCES
- 1. American Diabetes Association . Standards of medical care in diabetes – 2016. Diabetes Care. 2016;39(suppl 1):S1‐S112. [DOI] [PubMed] [Google Scholar]
- 2. Raccah D, Bretzel RG, Owens D, Riddle M. When basal insulin therapy in type 2 diabetes mellitus is not enough – what next? Diabetes Metab Res Rev. 2007;23:257‐264. [DOI] [PubMed] [Google Scholar]
- 3. Ahrén B, Gautier JF, Berria R, Stager W, Aronson R, Bailey CJ. Pronounced reduction of postprandial glucagon by lixisenatide: a meta‐analysis of randomized clinical trials. Diabetes Obes Metab. 2014;16:861‐868. [DOI] [PubMed] [Google Scholar]
- 4. Lorenz M, Pfeiffer C, Steinstrasser A, et al. Effects of lixisenatide once daily on gastric emptying in type 2 diabetes – relationship to postprandial glycemia. Regul Pept. 2013;185:1‐8. [DOI] [PubMed] [Google Scholar]
- 5. Meier JJ. GLP‐1 receptor agonists for individualized treatment of type 2 diabetes mellitus. Nat Rev Endocrinol. 2012;8:728‐742. [DOI] [PubMed] [Google Scholar]
- 6. Meier JJ, Rosenstock J, Hincelin‐Mery A, et al. Contrasting effects of lixisenatide and liraglutide on postprandial glycemic control, gastric emptying, and safety parameters in patients with type 2 diabetes on optimized insulin glargine with or without metformin: a randomized, open‐label trial. Diabetes Care. 2015;38:1263‐1273. [DOI] [PubMed] [Google Scholar]
- 7. Riddle MC, Forst T, Aronson R, et al. Adding once‐daily lixisenatide for type 2 diabetes inadequately controlled with newly initiated and continuously titrated basal insulin glargine: a 24‐week, randomized, placebo‐controlled study (GetGoal‐Duo 1). Diabetes Care. 2013;36:2497‐2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Riddle MC, Aronson R, Home P, et al. Adding once‐daily lixisenatide for type 2 diabetes inadequately controlled by established basal insulin: a 24‐week, randomized, placebo‐controlled comparison (GetGoal‐L). Diabetes Care. 2013;36:2489‐2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Aroda VR, Rosenstock J, Wysham C, et al. Efficacy and safety of LixiLan, a titratable fixed‐ratio combination of insulin glargine plus lixisenatide in type 2 diabetes inadequately controlled on basal insulin and metformin: the LixiLan‐L randomized trial. Diabetes Care. 2016;39:1972‐1980. [DOI] [PubMed] [Google Scholar]
- 10. Rosenstock J, Arranz C, Grunberger G, et al. Benefits of LixiLan, a titratable fixed‐ratio combination of insulin glargine plus lixisenatide versus insulin glargine and lixisenatide monocomponents in type 2 diabetes inadequately controlled on oral agents: the LixiLan‐O randomized trial. Diabetes Care. 2016;39:2026‐2035. [DOI] [PubMed] [Google Scholar]
- 11. Buse JB, Vilsbøll T, Thurman J, et al. Contribution of liraglutide in the fixed‐ratio combination of insulin degludec and liraglutide (IDegLira). Diabetes Care. 2014;37:2926‐2933. [DOI] [PubMed] [Google Scholar]
- 12. Lingvay I, Manghi FP, Garcia‐Hernandez P, et al. Effect of insulin glargine up‐titration vs insulin degludec/liraglutide on glycated hemoglobin levels in patients with uncontrolled type 2 diabetes: the DUAL V randomized clinical trial. JAMA. 2016;315:898‐907. [DOI] [PubMed] [Google Scholar]
- 13. Harris S, Jaeckel E, Jodar E, et al. Impact of BMI on HbA1c reduction in response to IDegLira in subjects with type 2 diabetes (T2D) uncontrolled on SU, GLP‐1RA or insuline glargine: analyses from completed phase 3 b trials. Diabetes. 2016;65(suppl 1):938‐P. [Google Scholar]
- 14. Rosenstock J, Shenouda SK, Bergenstal RM, et al. Baseline factors associated with glycemic control and weight loss when exenatide twice daily is added to optimized insulin glargine in patients with type 2 diabetes. Diabetes Care. 2012;35:955‐958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sorli C, Harris S, Jodar E, et al. IDegLira is efficacious across baseline HbA1c categories in subjects with type 2 diabetes uncontrolled on SU, GLP‐1RA or insulin glargine: analyses from completed phase 3b trials. Diabetes. 2016;65(suppl 1):925‐P. [Google Scholar]
- 16. Garber AJ. Treat‐to‐target trials: uses, interpretation and review of concepts. Diabetes Obes Metab. 2014;16:193‐205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ahrén B, Leguizamo DA, Miossec P, Saubadu S, Aronson R. Efficacy and safety of lixisenatide once‐daily morning or evening injections in type 2 diabetes inadequately controlled on metformin (GetGoal‐M). Diabetes Care. 2013;36:2543‐2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fonseca VA, Alvarado‐Ruiz R, Raccah D, Boka G, Miossec P, Gerich JE. Efficacy and safety of the once‐daily GLP‐1 receptor agonist lixisenatide in monotherapy: a randomized, double‐blind, placebo‐controlled trial in patients with type 2 diabetes (GetGoal‐Mono). Diabetes Care. 2012;35:1225‐1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Seino Y, Min KW, Niemoeller E, Takami A. Randomized, double‐blind, placebo‐controlled trial of the once‐daily GLP‐1 receptor agonist lixisenatide in Asian patients with type 2 diabetes insufficiently controlled on basal insulin with or without a sulfonylurea (GetGoal‐L‐Asia). Diabetes Obes Metab. 2012;14:910‐917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yu Pan C, Han P, Liu X, et al. Lixisenatide treatment improves glycaemic control in Asian patients with type 2 diabetes mellitus inadequately controlled on metformin with or without sulfonylurea: a randomized, double‐blind, placebo‐controlled, 24‐week trial (GetGoal‐M‐Asia). Diabetes Metab Res Rev. 2014;30:726‐735. [DOI] [PubMed] [Google Scholar]


