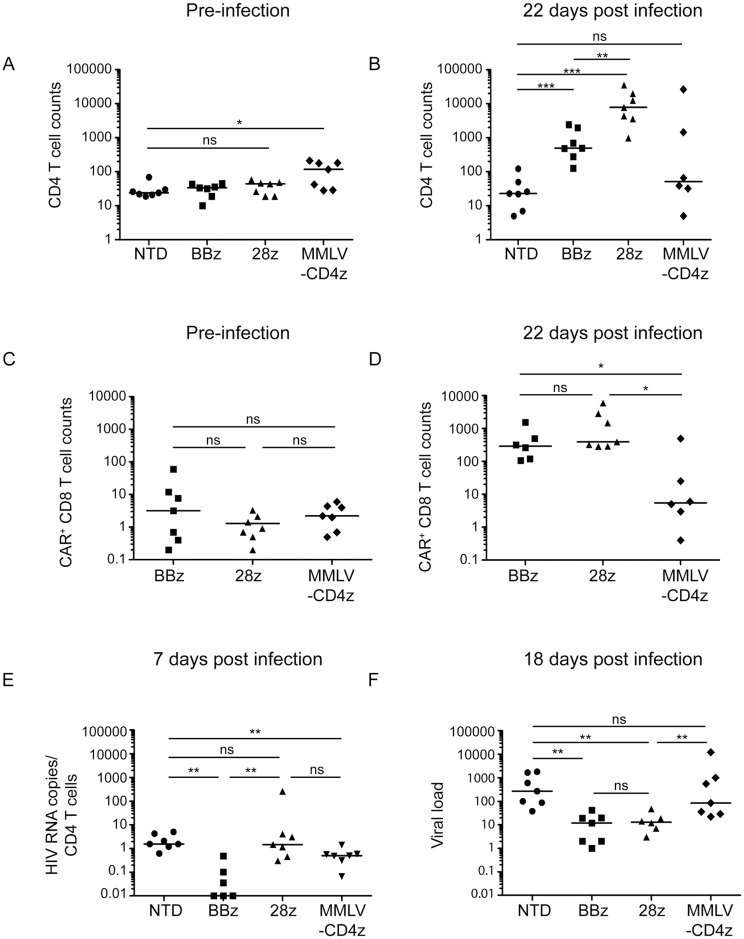
Fig 7. Optimized CAR T cells control HIV-1 replication better and expand to greater levels in vivo than first generation CAR T cells.
Cohorts of NSG mice were infused with 8 million human CD4 T cells and 2 million human CD8 T cells (50% CAR transduction efficiency). CD8 T cells were either left NTD, transduced with optimized CD4 CARs containing either 4-1BB or CD28 intracellular costimulatory domains, or the clinical trial, MMLV-based CAR, denoted in as NTD, BBz, 28z, and MMLV-CD4z, respectively. Three weeks post injection, engraftment was measured to determine (A) baseline peripheral CD4 T cell counts and (C) baseline CAR+ CD8 T cell counts. Two days later mice were infected with HIV-1 Bal via tail vein injection. 22 days post infection, (B) endpoint peripheral CD4 T cell counts and (D) CAR+ CD8 T cell counts were obtained. (E) Seven and (F) eighteen days post infection mice were bled and HIV RNA copies per μl plasma were determined by qPCR and normalized to CD4 T cell counts. The non-normalized viral loads are displayed in S8 Fig. Mann Whitney Test was used to determine statistical significance (p values: ns >0.05, *<0.05, **<0.01, ***<0.0001).