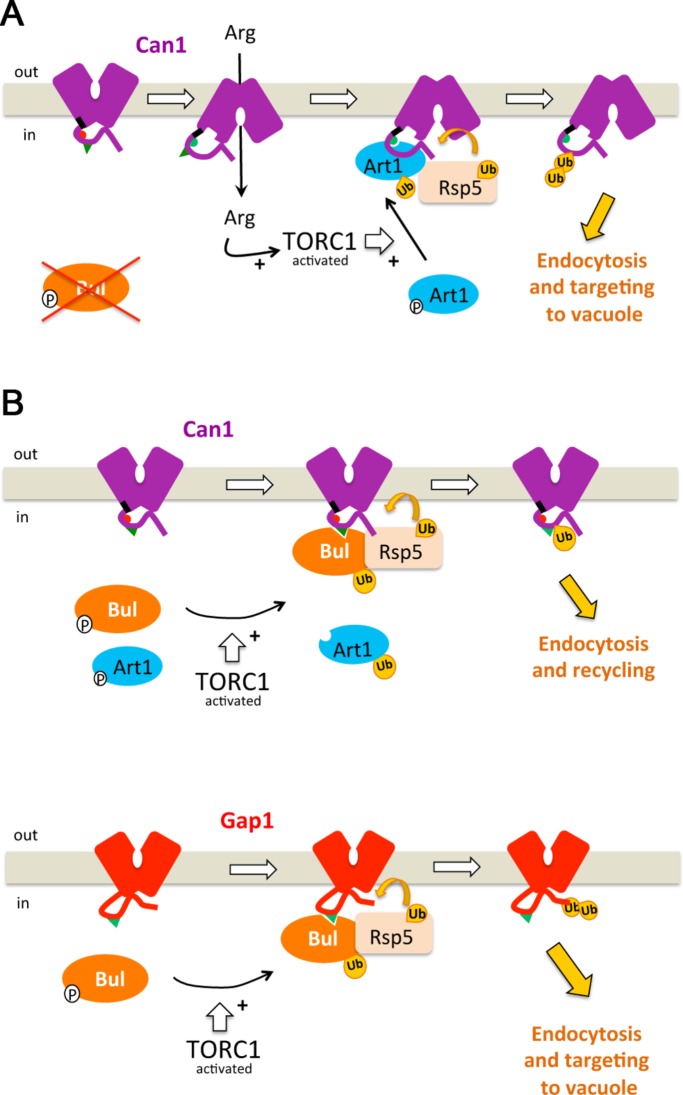
FIGURE 9:
Model of the mechanisms governing ubiquitylation and endocytosis of yeast amino acid permeases. (A) Art1- and Rsp5-mediated ubiquitylation and down-regulation of Can1 permease in response to substrate transport and TORC1 activation, as in the mutant lacking the Bul α-arrestins, for example. In the absence of substrate, the short ELK sequence (black) close to TM1 is oriented toward the core of the transporter and interacts with cytoplasmic loops, structuring the remaining tail so that the binding site for Art1 (aa 70–81, red hemicycle) is masked. In the presence of Arg, a transient switch of Can1 to an IF conformation, via repositioning of TM1 and the ELK tripeptide, causes a structural rearrangement unmasking the binding site for Art1 (now green). Arg uptake also stimulates TORC1, which activates Art1. Once activated, Art1 can recognize the unveiled binding site. This results in Rsp5-mediated Can1 ubiquitylation, mainly on Lys-42 or Lys-45. (B) Ubiquitylation and endocytosis of Gap1 and Can1 in response to TORC1 stimulation. Activation of TORC1 stimulates the Art1 and Bul α-arrestins. Once activated, the Buls can act through a permanently exposed region of the Gap1 N-tail (residues 20–35), causing its ubiquitylation on Lys-9 or Lys-16. This modification efficiently targets Gap1 to the vacuole. The activated Buls also recognize a permanently exposed binding site in the Can1 N-tail (residues 62–69), causing its ubiquitylation. This modification does not efficiently target the internalized Can1 to the vacuole. The TORC1-activated Art1 does not efficiently recognize Can1 because its binding site remains masked.