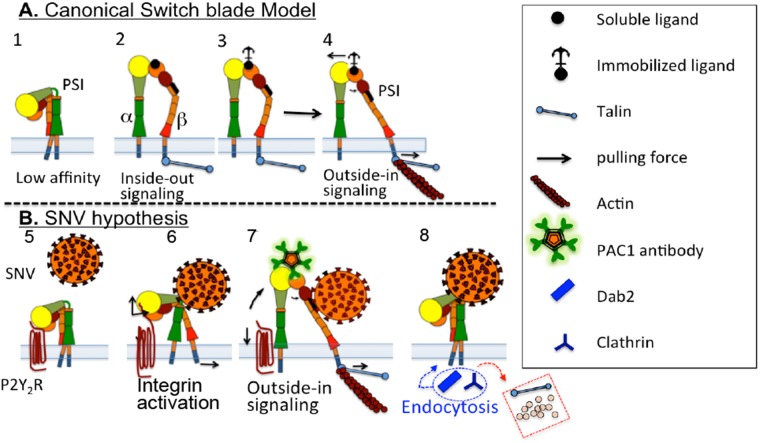
FIGURE 1:
Switchblade model for integrin activation (adapted from Schurpf and Springer [2011]) and schematic presenting the hypothesis that cis interaction of αIIbβ3 integrin and RGDP2Y2R mediates integrin activation initiated by binding of SNV to the PSI domain. (A) 1) Structure of an inactive integrin. 2–3) Intracellular signaling (inside-out) induces integrin activation mediated by binding of adaptor proteins (such as talin) to the extended conformation with open head-piece bound to soluble and immobilized ligands (see the text for details). 4) Development of mechanochemical force selectively transduced through the β subunit. Integrin binding to immobilized ligand resists lateral translation and causes an increase in force (indicated by arrows) and promotes separation of the α− and β-subunit transmembrane domains. (B) 5) P2Y2R interacts in cis with αIIbβ3 integrin. 6) SNV occupancy of the PSI domain induces an increase in integrin affinity for cis-RGDP2Y2R (based on AFM measurements). Integrin extension exerts a membrane normal/lateral pulling force, due to height differences between the membrane proximal RGDP2Y2R, and the unbending integrin (Takagi et al., 2002; Chigaev et al., 2003, 2015); Outside-in signaling. 7) Recruitment of talin and other adhesion molecules increases force transduction, which is terminated by rupture of RGD interaction. Full extension of αIIbβ3 integrin causes rupture of cis interaction, indicated by PAC1 staining of cells. 8) Cessation of tensile force, and loss of intracellular link to actin, leads to exchange of adhesion proteins, which are replaced by adaptor proteins (e.g., clathrin and Dab2) for integrin endocytosis (Yu et al., 2015).