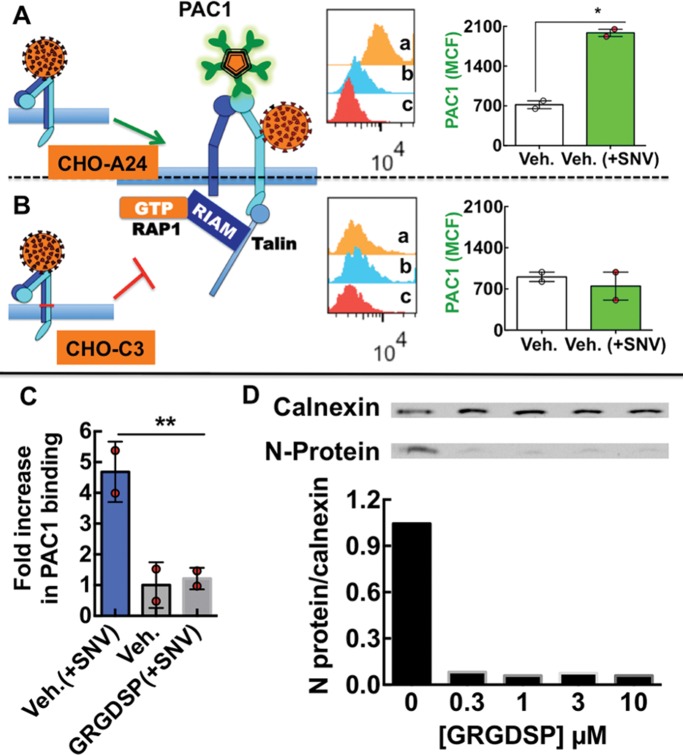
FIGURE 9:
SNV binding to integrin PSI domain recapitulates physiological activation of αIlbβ3. (A, B) Model of SNV induced extended conformation associated with a high-affinity state with separated α and β transmembrane and cytoplasmic domains, which allows the ligand mimetic monoclonal anti-αIlbβ3 antibody PAC1 to bind to CHO-A24 cells stably expressing wild-type αIIbβ3. Flow cytometry histogram inserts show fluorescence associated with (a) PAC-1 staining of 50,000 cells activated with SNV particles, (b) staining of resting cells, and (c) autofluorescence of unstained cells. The graph is a plot of PAC-1 staining (1:20 dilution) of CHO-A24 corrected for autofluorescence. Cells were exposed to SNV for 5 min at 37°C and then quenched on ice, fixed with 3% paraformaldehyde for 10 min, and stained with PAC1 at 1:20 dil. for 30 min on shaker at 37°C. Poor PAC1 staining of CHO-C3 cells expressing integrin mutant with an inner membrane clasp at the αIIbW968C/β3I693, which prevents subunit separation. Experiments were performed in parallel with CHO-A24. Error bar is SE for duplicate measurements, *p < 0.05. (C) Plot of fold increase over resting cells in PAC-1 staining of CHO-A24 cells in suspension after 30 min exposure to killed SNV. 10,000 CHO-A24 cells suspended in 20 µl media containing 1:10 dilution PAC-1 antibody, coadministered with 1 µM GRGDSP. (D) Titration of soluble GRGDSP peptide, which abrogates cis-interaction of the integrin and RGDP2Y2R, inhibits infection of suspension CHO-24 cells. CHO-A24 cells were infected in suspension with 0.1 moi SNV for 30 min. Cells were then washed in low pH media three times and then incubated in normal media for 24 h and then assayed for viral N-protein.