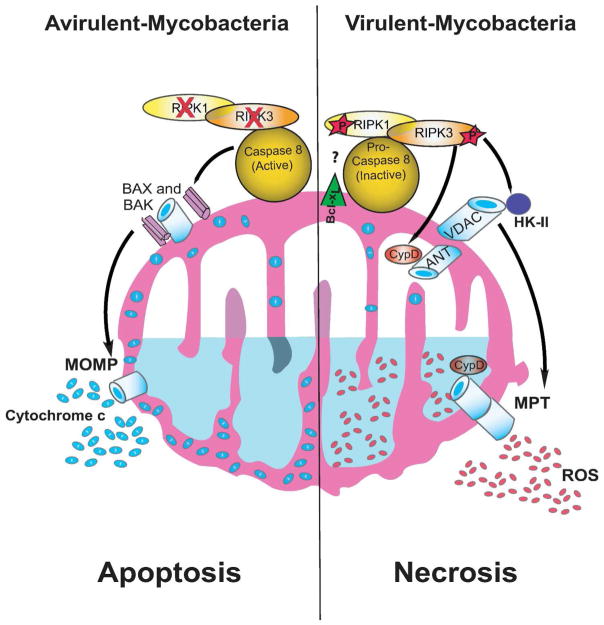
Figure 8. Model of RIPK3-dependent programmed necrosis and caspase 8-dependent apoptosis in Mφ infected with Mtb.
In Mφ infected with virulent Mtb RIPK3 and pro-caspase 8 present in the cytosol translocate to the mitochondria in presence of Bcl-xL and RIPK3 is activated RIPK3 enhances binding of HKII to VDAC on the outer mitochondrial membrane controlling mitochondrial glycolysis. At the same time activated RIPK3 triggers CypD-dependent formation of the mitochondrial permeability transition (MPT) pore via interaction between ANT and VDAC leading to leakage of the electron chain. Both mechanisms seem to be required for increasing ROS-dependent necrosis (right). In Mφ infected with avirulent Mtb the RIPK3 and caspase 8 also translocate to the mitochondria but this step is quickly followed by activation of caspase 8 and degradation of RIPK3. Oligomerization of BAX and BAK, which in turn allows the release of pro-apoptotic molecules (e.g. cytochrome c) leads to apoptosis (left). The exact action mechanism of Bcl-xL function is unknown.