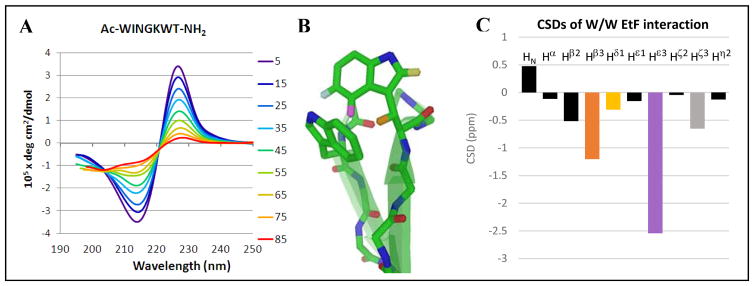
Fig. 2.
A) The CD melt for a minimized turn-flanking W/W interaction; in this sequence (Ac-WINGKWT-NH2), the bisignate exciton couplet is essentially the only feature evident in the CD spectrum; the fraction folded derived for this peptide motif was χF = 0.76 at the lowest temperature examined. Adjusting to 100% folded, [θ]228 = +460,000, a typical value for this interaction. B) The EtF indole/indole cluster geometry, which gives rise to a 227–229 nm ellipticity maximum in the CD spectrum, also produces upfield ring current shifts at the edge-Trp. Diagnostic protons are color coded and labeled here and in panel C. C) The structuring shifts of the edge-Trp, given as chemical shift deviations (CSDs) from statistical coil norms, typical values (ppm) are: Hβ3 (orange, −1.4 ± 0.45), Hε3 (lavender, −2.25 ± 0.40), Hζ3 (light gray, −0.65 ± 0.15), and Hδ (yellow, −0.45 ± 0.2).