History
Before the advent of MRI, the diagnosis of a rotator cuff tear was difficult and largely relied on physical examination and plain radiographs. Several radiographic findings were associated with rotator cuff tears, including cystic appearance of the upper 2/3 of the anatomic neck, sclerosis and atrophy of the greater tuberosity, notching between the articular surface and greater tuberosity, irregular new bone formation on the lateral margin of the acromion, and a decrease in the acromiohumeral interval (AHI). Golding [2] was the first to report the AHI variation of 6 mm to 14 mm in 150 normal subjects. Series from Cotton and Rideout [1] and Weiner and Macnab [14] reiterated these previous findings and emphasized that an AHI less than 6 or 7 mm correlated with a rotator cuff tear. They noted, however, that this finding was not completely reliable. Weiner and Macnab [14] first postulated that the imbalance between the superiorly directed force of the deltoid and lack of the humeral head depressing force normally generated by the supraspinatus allowed the humeral head to migrate superiorly, resulting in this decreased AHI [14]. Although such radiographic findings were suggestive of a rotator cuff tear, the diagnosis often was uncertain and only verified by surgical exploration. Diagnosing a tear by radiographs was considered difficult enough; radiographs shed little light on tear location, size, or severity.
In 1977, Neer et al. [8, 9] were the first to describe the term cuff tear arthropathy (CTA). This term, however, refers only to end-stage changes associated with massive rotator cuff tears when collapse of the subchondral bone of the humeral head and advanced arthritis are present. Neer et al. [8] proposed biological and mechanical factors were responsible for the development of CTA. They proposed that leaking of the synovial fluid from the massive rotator cuff tear resulted in reduced perfusion of nutrients into the articular cartilage. This nutritional deficiency to the cartilage, combined with gross instability of the humeral head, resulted in injury of the articular surfaces on the glenoid and humeral head [8]. Despite previous descriptions of radiographic findings consistent with massive rotator cuff tears and CTA [1, 14], there were no published studies at that time that had documented the radiographic natural history of massive rotator cuff tears. Although a few investigators had made reference to the pathomechanics of massive rotator cuff tears [14], before the study by Hamada et al. [3] there was no detailed description of the pathomechanics relating to different stages of massive rotator cuff tears as they were seen radiographically.
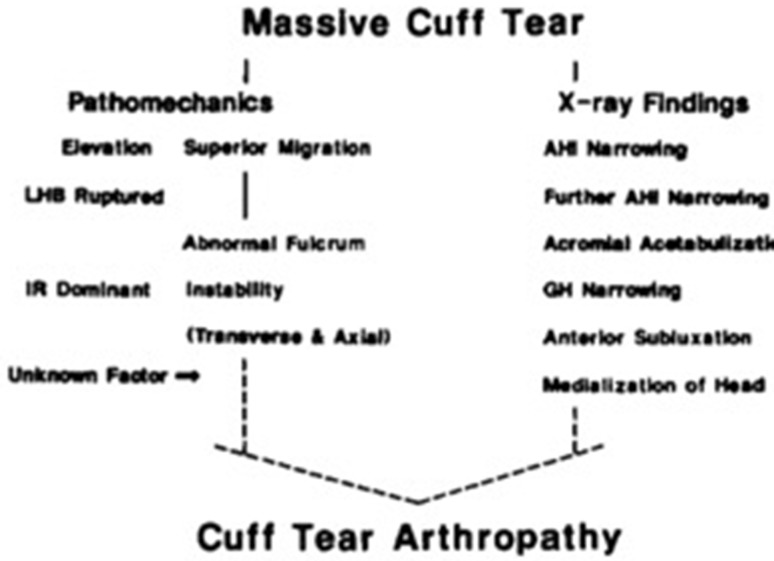
Hamada et al. [3] first described the radiographic findings of massive rotator cuff tears in 1990. This became the first account of the progression of radiographic findings in massive cuff tears. They suggested that massive rotator cuff tears will eventually progress to CTA through a set of pathomechanics corresponding to specific findings seen on radiographs (Fig. 1) [3]. First, deltoid contraction during forward elevation causes superior migration of the humeral head and a decrease in the AHI. Without the counterforce of the rotator cuff to depress the humeral head in the center of the glenoid, they theorized that there is increased demand transferred to the long head of the biceps as a humeral head depressor. Increased stress across this tendon along with mechanical friction seen between the superior humeral head and acromial undersurface can lead to rupture of the long head of the biceps tendon, further narrowing the AHI. As the superior migration continues, the humeral head abuts the acromion or the coracoacromial ligament, which now acts as a fulcrum leading to acromial acetabulization. With massive rotator cuff tears, the weakness in external rotation causes further instability of the glenohumeral joint in the transverse and axial planes, leading to glenohumeral joint narrowing, medialization, and anterior subluxation, culminating in CTA (Table 1).
Fig. 1.
The original description from Hamada et al. [3] depicting the pathomechanics leading to radiographic changes in massive rotator cuff tears. IR = internal rotation; GH = glenohumeral. (Published with permission from Wolters Kluwer from Hamada K, Fukuda H, Mikasa M, Kobayashi Y. Roentgenographic findings in massive rotator cuff tears: a long-term observation. Clin Orthop Relat Res. 1990;254:92–96.).
Table 1.
Status of the acromiohumeral space, glenohumeral joint, rotator cuff, and long head of the biceps according to grade in Hamada classification [3]
| Grade | Acromiohumeral space | Glenohumeral joint | Rotator cuff/LHB tear |
|---|---|---|---|
| 1 | AHI > 6 mm | Normal | Massive with LHB intact |
| 2 | AHI < 5 mm | Normal | Massive with LHB tear |
| 3 | Acetabulization | Normal | Massive with LHB tear |
| 4 | Acetabulization | Narrowed | Massive with LHB tear |
| 5 | Acetabulization | Humeral head collapse | Massive with LHB tear |
AHI = acromiohumeral interval; LHB = long head of the biceps.
Purpose
The purpose of the Hamada classification is to provide a mechanistic explanation to the radiographic findings in massive rotator cuff tears. It was through the classification that Hamada et al. theorized that all massive rotator cuff tears eventually will lead to CTA and will progress through certain stages with an accompanying set of radiographic features.
In the case of CTA, various soft tissue structures help stabilize the glenohumeral joint. The most important of these are the rotator cuff tendons. When the rotator cuff is injured, other soft tissue structures such as the long head of the biceps and coracoacromial ligament help restrain humeral head migration. Eventually, as CTA progresses, these soft tissues can fail resulting in permanent changes to the bony structure of the shoulder. The classification scheme was proposed by Hamada et al. to identify the progressive nature of massive rotator cuff tears with the end stage being CTA. The system was designed to help determine the status of the rotator cuff and associated soft tissue structures as they are affected in various stages of a massive rotator cuff tear, which potentially could help guide the surgeon to a preoperative plan for exposure, surgical technique, and reconstruction.
Description
From 1976 to 1983, Hamada et al. [3] acquired 65 complete sets of arthrograms before operating on rotator cuff tears. Each set of arthrograms included four radiographic views: a scapular-Y view and three AP views with the arm in 30° external rotation, neutral rotation, and 60° internal rotation. They excluded the scapular-Y views because none of the reviewed arthrogram sets showed a subscapularis tear. This left the classification to be based on three AP-view arthrograms with the arm in various rotations. They classified these arthrograms in four groups.
In Group 1 arthrograms, the humeral head and subacromial bursa were not in continuity in any of the three projections (that is, rotations) and were suggestive of small tears of the supraspinatus tendon. Group 2 arthrograms had the contours continuous in external rotation only and were indicative of a medium-size tear of the supraspinatus. When the humeral head and subacromial bursa became continuous on the external and neutral rotation views, they were classified as Group 3 and thought to represent a massive tear (supraspinatus and infraspinatus). Finally, when the contours were in continuity on all three views, they were classified as Group 4 and represented global rotator cuff tears. While confirming the arthrograms surgically, Hamada et al. [3] found small tears of the supraspinatus in 85% of Group 1 arthrograms, medium tears of the supraspinatus in 79% of Group 2 arthrograms, and massive tears seen in 86% of Group 3 arthrograms. These findings became the basis on how they would identify patients with massive rotator cuff tears who were treated nonoperatively, and who ultimately were used for the classification scheme.
Hamada et al. [3] then combined Groups 3 and 4 to represent massive rotator cuff tears and matched these radiographic findings with those of 22 patients who were treated nonoperatively for massive tears. These patients were followed radiographically and became the basis of the Hamada classification for massive rotator cuff tears.
The Hamada classification of massive rotator cuff tears is largely based on the AHI, which previously was considered a sensitive marker for a full-thickness rotator cuff tear [1, 14]. Previous studies [1, 14] had set the lower limits of normal AHI at 6 mm to 7 mm, which became the distinction between a Grade 1 and Grade 2 massive rotator cuff tear in the Hamada classification system. Grade 1 represented preserved AHI or greater than 6 mm. In Grade 2, the AHI was 5 mm or less. Grade 3 was further characterized by the addition of a concave deformity to the acromial undersurface, or “acromial acetabulization.” This acetabulization can occur in one of two different subtypes: one being “an excavating deformity of the acromion” and the other caused by excessive spurring along the coracoacromial ligament. In Grade 4, narrowing of the glenohumeral joint was added to the Grade 3 features. Finally, Grade 5 was characterized by humeral head collapse and termed CTA (Fig. 2).
Fig. 2A–E.
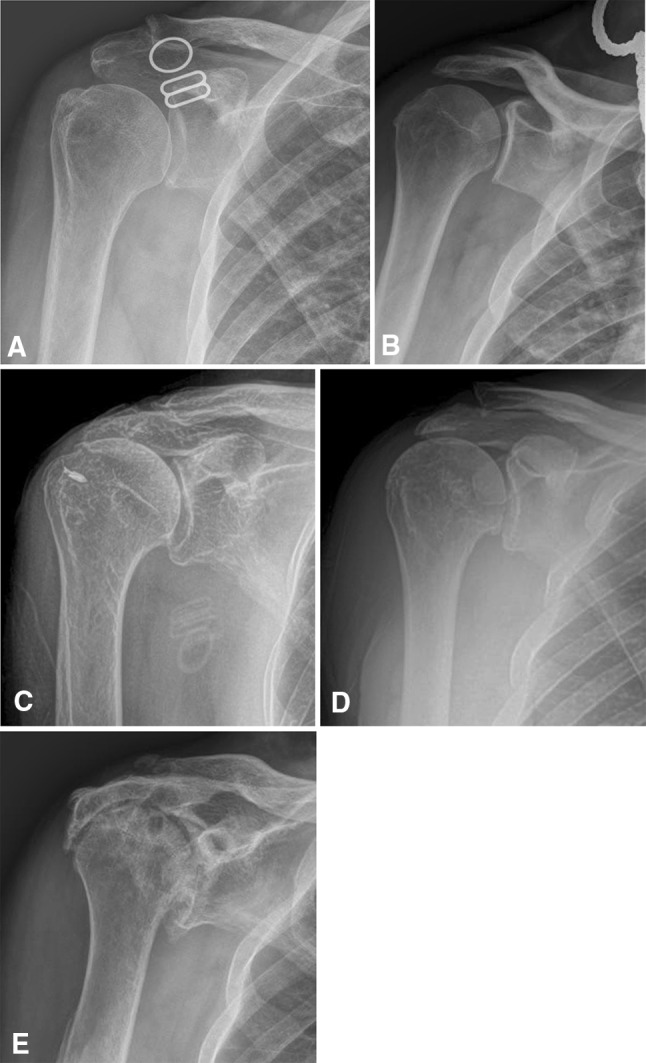
The radiographs show the different stages of Hamada’s classification of massive rotator cuff tears. (A) Grade 1 is characterized by a preserved acromiohumeral interval. (B) Grade 2 shows an acromiohumeral interval less than 5 mm. (C) Grade 3 shows the development of acromial acetabulization. (D) Grade 4 shows the addition of glenohumeral narrowing, and (E) Grade 5 is characterized by the development of humeral head collapse, and is termed cuff tear arthropathy.
In 2005, Nove-Josserand et al. [10] and Walch et al. [13] identified patients who had glenohumeral narrowing without acromial acetabulization. This led Walch’s group to divide the Hamada Grade 4 classification into two subtypes [13]. In Grade 4A, they noted narrowing of the glenohumeral joint without subacromial acetabulization. In Grade 4B, the glenohumeral joint space displays narrowing in the setting of subacromial acetabulization.
Reliability
One validation study that included four fellowship-trained shoulder surgeons who evaluated 45 sets of shoulder radiographs in patients with CTA found intraobserver kappa values ranging from 0.72 to 1.0 and interobserver kappa value of 0.429 when evaluating images that were noted to be of good quality [4].
A study including images of 52 shoulders with CTA as classified by an orthopaedic surgeon and a senior orthopaedic resident showed intraobserver reliability kappa value of 1.0 and 0.491 for each reviewer, and interobserver reliability kappa value of 0.407 [5]. Kappe et al. [5] concluded that the Hamada classification possessed sufficient reliability to be used in everyday practice and for scientific purposes.
Limitations
Although the Hamada classification does include glenohumeral joint degeneration, it does not address morphologic changes in the glenoid. These alterations are better defined with the classification described by Sirveaux et al. [11], a scheme that also has been shown to correlate with postoperative scapular notching. Their classification defines four types of glenoid erosion. Type E0 has proximal migration of the humeral head without erosion of the glenoid. Type E1 is defined by concentric erosion, Type E2 with erosion of the superior part of the glenoid, and Type E3 has erosion that extends to the inferior portion of the glenoid. Additionally, although the Hamada classification does differentiate shoulders for which a joint-preserving procedure would be recommended from those which would be better managed with arthroplasty, it does not provide a basis to select a type of prosthesis like with the Seebauer classification described by Visotsky et al. [12]. The Seebauer classification defines four groups distinguished by the degree of superior migration from the center of rotation and the amount of instability of the center of rotation. The classification is based on biomechanics of the joint to aid in decision-making of implant type and goals of reconstruction. This has led some authors to suggest that the Hamada classification may be more useful during early stages of CTA, whereas the later stages may be better addressed by the Seebauer classification [5]. In particular, the Hamada classification does not specifically address anterosuperior escape like in the Seebauer classification [3, 12]. This is the primary reason that surgeons have been reported to have a strong preference for the Seebauer classification, again for its aid in clinical decision-making of hemiarthroplasty versus reverse shoulder arthroplasty, however the usefulness of this today is limited [4].
Walch et al. [13] recognized a group of patients with glenohumeral joint narrowing without acromial acetabulization who were not categorized well with the Hamada classification. To address this, they divided Grade 4 of the Hamada classification into two subgroups as previously described. In another study, Nové-Josserand et al. [10] found that there was not a consistent linear progression through the Hamada grading system. In particular, they criticized that the Hamada grading did not provide adequate explanation for glenohumeral degradation [10].
Like with all radiographic-based classification schemes, the Hamada classification is limited by the quality of the radiographs. Projection of the radiographs and position of the arm can affect the appearance of glenohumeral narrowing and measurement of AHI. Iannotti et al. [4] reported that average interrater correlation coefficients increased from 0.184 to 0.429 with the distinction of poor-quality versus good-quality radiographs. Similar ranges were seen with the Favard [11] and Seebauer classifications [12], depending on image quality as well. Although the Hamada classification has shown intraobserver and interobserver reliability similar to other classifications, these values overall remain low.
In addition, some authors [6, 7] suggest that radiograph-based classification schemes lack consistent clinical relevance. Although Nové-Josserand et al. [10] found a correlation between the Hamada stage and the Constant score, a study of 307 patients was unable to find predictive information regarding a patient’s clinical status using conventional AP radiographs alone, from which the Hamada grade is determined [7]. This lack of clinical correlation has led some authors to create classification schemes based on clinical symptoms [6].
Two major advances have limited the clinical usefulness of the Hamada classification today. The first is the routine use of MRI in the diagnosis of rotator cuff tears. MRI has allowed the surgeon to gather much more preoperative information regarding the size of the tear, degree of retraction, quality of the rotator cuff muscle bellies, and status of the articular cartilage. This has made radiographs obsolete in the diagnosis of a rotator cuff tear, which the Hamada classification is based on. The second is the use of the reverse shoulder arthroplasty in the treatment of massive rotator cuff tears. Reverse shoulder arthroplasty has limited the indication for hemiarthroplasty in the setting of CTA to use in severe glenoid bone loss when a glenoid component is unable to be implanted. This has made the status of supporting soft tissue structures such as the coracoacromial ligament and subscapularis tendon less important.
Conclusions
The Hamada classification is a commonly used classification scheme that uses a mechanistic approach to explain the radiographic changes seen with chronic massive rotator cuff tears. It highlights the progressive nature of massive rotator cuff tears leading to CTA and is based on AHI, presence of acromial acetabulization, and finally glenohumeral arthritis with humeral head collapse. With good-quality radiographs, it has fair inter- and intraobserver reliability. Unfortunately, the clinical utility of the Hamada classification is lacking given the widespread use of MRI and reverse shoulder arthroplasty, and its lack of characterizing glenoid-sided bony erosion, which may be useful in deciding whether bone graft or an augmented component is necessary. The treatment of massive rotator cuff tears today largely relies on patient factors including age and activity level, and on tendon and muscle quality, retraction, and presence of significant arthritis, making a pure radiographic classification scheme outdated. Even though the single grades of the classification do not necessarily guide treatment, general concepts of the classification such as the presence of arthrosis, as in Grades 4 and 5, lead surgeons toward reverse shoulder arthroplasty, while Grade 1 or 2 may be amendable to joint-preserving operations. It is important to remember the Hamada classification from a historical perspective as it showed the progressive nature of untreated massive rotator cuff tears and tying the pathomechanics to specific radiographic features. Today, however, we have seen a decline in its clinical relevance and it largely remains historical in perspective.
Footnotes
Each author certifies that neither he, nor any member of his immediate family, have funding or commercial associations (consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
References
- 1.Cotton RE, Rideout DF. Tears of the humeral rotator cuff: a radiological and pathological necropsy survey. J Bone Joint Surg Br. 1964;46:314–328. [PubMed] [Google Scholar]
- 2.Golding FC. The shoulder: the forgotten joint. Br J Radiol. 1962;35:149–158. doi: 10.1259/0007-1285-35-411-149. [DOI] [PubMed] [Google Scholar]
- 3.Hamada K, Fukuda H, Mikasa M, Kobayashi Y. Roentgenographic findings in massive rotator cuff tears: a long-term observation. Clin Orthop Relat Res. 1990;254:92–96. [PubMed] [Google Scholar]
- 4.Iannotti JP, McCarron J, Raymond CJ, Ricchetti ET, Abboud JA, Brems JJ, Williams GR. Agreement study of radiographic classification of rotator cuff tear arthropathy. J Shoulder Elbow Surg. 2010;19:1243–1249. doi: 10.1016/j.jse.2010.02.010. [DOI] [PubMed] [Google Scholar]
- 5.Kappe T, Cakir B, Reichel H, Elsharkawi M. Reliability of radiologic classification for cuff tear arthropathy. J Shoulder Elbow Surg. 2011;20:543–547. doi: 10.1016/j.jse.2011.01.012. [DOI] [PubMed] [Google Scholar]
- 6.Loew M, Raiss P. A symptom-based classification for shoulders with massive rotator cuff defects. Int Orthop. 2010;34:63–69. doi: 10.1007/s00264-009-0725-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Middernacht B, Winnock de Grave P, Van Maele G, Favard L, Molé D, De Wilde L. What do standard radiography and clinical examination tell about the shoulder with cuff tear arthropathy? J Orthop Surg Res. 2011;6:1. [DOI] [PMC free article] [PubMed]
- 8.Neer CS, 2nd, Craig EV, Fukuda H. Cuff-tear arthropathy. J Bone Joint Surg Am. 1983;65:1232–1244. doi: 10.2106/00004623-198365090-00003. [DOI] [PubMed] [Google Scholar]
- 9.Neer CS, 2nd, Cruess RL, Sledge CS, Wilde AH. Total glenohumeral replacement: a preliminary report. Orthop Trans. 1977;1:244–245. [Google Scholar]
- 10.Nové-Josserand L, Walch G, Adeleine P. Courpron P [Effect of age on the natural history of the shoulder: a clinical and radiological study in the elderly][in French] Rev Chir Orthop Reparatrice Appar Mot. 2005;91:508–514. doi: 10.1016/S0035-1040(05)84440-X. [DOI] [PubMed] [Google Scholar]
- 11.Sirveaux F, Favard L, Oudet D, Huquet D, Walch G, Mole D. Grammont inverted total shoulder arthroplasty in the treatment of glenohumeral osteoarthritis with massive rupture of the cuff: results of a multicentre study of 80 shoulders. J Bone Joint Surg Br. 2004;86:388–395. doi: 10.1302/0301-620X.86B3.14024. [DOI] [PubMed] [Google Scholar]
- 12.Visotsky JL, Basamania C, Seebauer L, Rockwood CA, Jensen KL. Cuff tear arthropathy: pathogenesis, classification, and algorithm for treatment. J Bone Joint Surg Am. 2004;86(suppl 2):35–40. doi: 10.2106/00004623-200412002-00007. [DOI] [PubMed] [Google Scholar]
- 13.Walch G, Edwards B, Boulahia A, Nove-Josserand L, Neyton L, Szabo I. Arthroscopic tenotomy of the long head of the biceps in the treatment of rotator cuff tears: clinical and radiographic results of 307 cases. J Shoulder Elbow Surg. 2005;14:238–246. doi: 10.1016/j.jse.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 14.Weiner DS, Macnab I. Superior migration of the humeral head: a radiological aid in the diagnosis of tears of the rotator cuff. J Bone Joint Surg Br. 1970;52:524–527. [PubMed] [Google Scholar]