Figure 1. Main components of the endoplasmic reticulum (ER) unfolded protein response (UPR) in mammals.
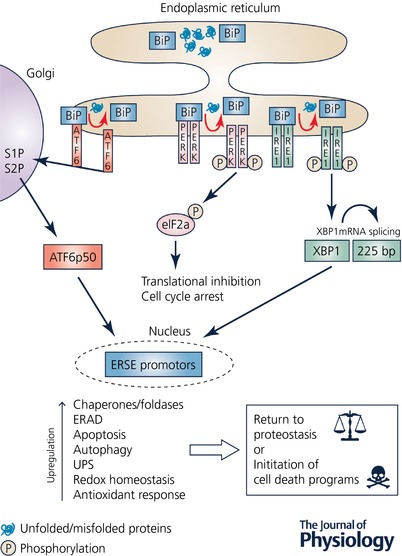
The intracellular build‐up of unfolded/misfolded proteins results in ER stress that initiates an adaptive response – the UPR – which invokes a cellular cascade in an attempt to return the cell back to proteostasis. The proximal UPR sensors are PKR‐like ER kinase (PERK), inositol requiring element‐1 (IRE1), and activating transcription factor 6 (ATF6). Following ER stress, BiP/GRP78 disassociates from PERK, IRE1 and ATF6, resulting in the initiation of the UPR, and also allows BiP itsel to undertake various chaperone activities. Activated PERK results in the phosphorylation of eukaryotic initiation factor 2α (eIF2α) that leads to inhibition of translation and cell cycle arrest, thus reducing protein loading within the cell. IRE1 splices a 225 base pair intron from its substrate XBP1, thus activating XBP1, which translocates to the nucleus and binds to specific endoplasmic reticulum stress elements (ERSE) within the nucleus resulting in the upregulation in expression of multiple genes, including cellular chaperones and foldases. Upon disassociation from Bip, ATF6 is cleaved by site‐1 protease (S1P) and site‐2 protease (S2P) within the Golgi apparatus to an active form ATF6 p50, which then translocates to the nucleus and induces endoplasmic reticulum associated protein degradation (ERAD) by the ubiquitin–proteasome system (UPS). If the ER stress is prolonged or severe and the UPR cannot return the cell to proteostasis, then cell death programmes, including the apoptosis cascade, will be initiated in order to remove the damaged cells.
