Figure 1. Selection of TMD mutants of GluA2.
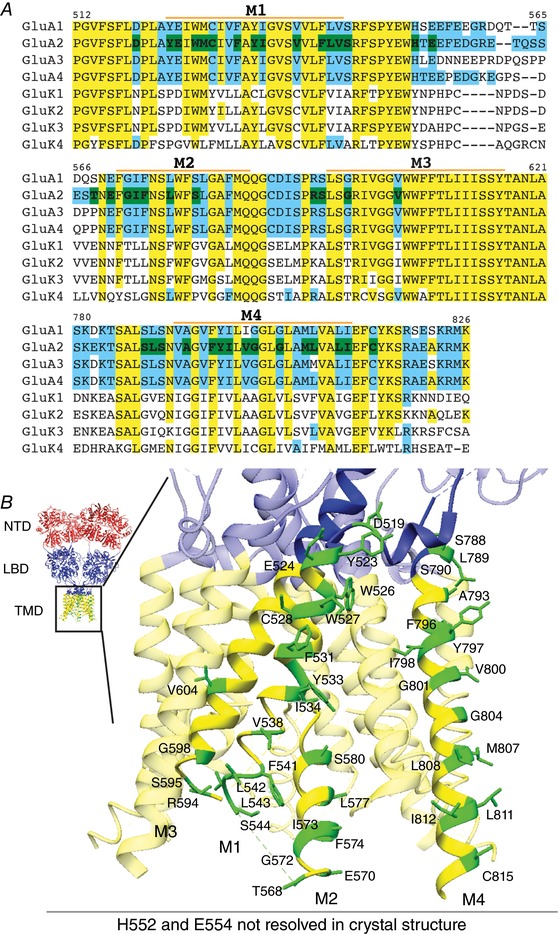
A, CLUSTALW alignment of M1–M4 of AMPARs and KARs. Yellow: conserved residues between GluA2 and GluK2; blue: residues not conserved between GluA2 and GluK2; green: residues of GluA2 mutated in this study. Residues different between GluA2 and GluK2 in the TMD regions, as well as bulky aromatic residues in the TMD were mutated. M1–4 labels indicate the extent of each transmembrane helix. B, GluA2 TMD residues that were studied in this report are indicated on the crystal structure (PDB: 4U4G). The domains are coloured red for the N‐terminal domain (NTD), blue for the ligand‐binding domain (LBD) and LBD–TMD linkers, and yellow for the transmembrane domain (TMD). All mutated residues are coloured green and labelled in the zoomed view of one GluA2 subunit. H522 and E554 are unresolved in the crystal structure and not shown, but occur on the intracellular linker between M1 and M2.
