Table 1. Selected Optimization Data for the Cu-Catalyzed Alkylation of Enamide 1a with EtMgBra.
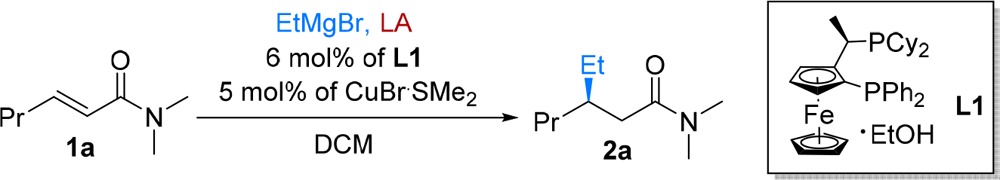
| Entry | L1/Cu(I) | LA | T [°C ] | Conv. [%]b | ee [%]c | Entry | L1/Cu(I) | LA | T [°C] | Conv. [%]b | ee [%]c | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | – | – | –78 | 0 | – | 7 | – | – | –50 | 12 | 0 | |
| 2 | Cu(I) | – | –78 | 0 | – | 8 | L1/Cu(I) | – | –50 | 20 | 5 | |
| 3 | L1/Cu(I) | – | –78 | 0 | – | 9 | – | BF3·Et2O | –78 | 0 | – | |
| 4 | – | – | 0 | 97 | – | 10 | – | TMSOTf | –78 | 50 | – | |
| 5 | Cu(I) | – | 0 | 42 | 0 | 11 | L1/Cu(I) | BF3·Et2O | –78 | 94 | 97 | |
| 6 | L1/Cu(I) | – | 0 | 79 | 0 | 12 | L1/Cu(I) | TMSOTf | –78 | 92 | 92 |
Reaction conditions: 0.1 M of 1a in CH2Cl2, LA (2.0 equiv), EtMgBr (2.0 equiv). For details see SI.
Conversion was determined by NMR of reaction crude.
Enantiomeric excess was determined by HPLC on a chiral stationary phase. Absolute configuration was assigned by analogy with literature data (see SI).
