Figure 14.
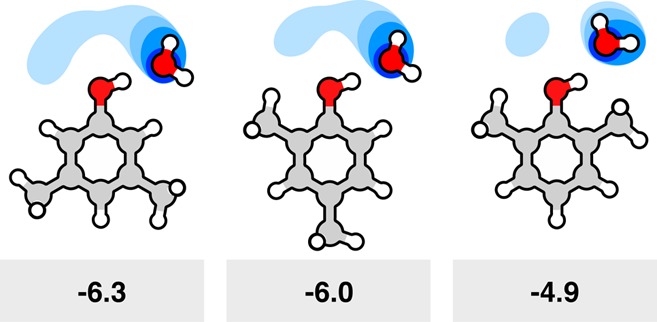
Solvation free energies can be nonadditive because the solute can perturb waters away from their cage-like favored structures. In a series of xylenol molecules, the different methyl group arrangements perturb the water solvation of the (red) hydroxyl group of each solute, particularly in the first solvation shell. The (blue) water occupancy from molecular simulations shows how displacing water from the ideally H-bonded structure leads to a decrease in the ΔGsolv (here in units of kcal mol–1).
