Figure 17.
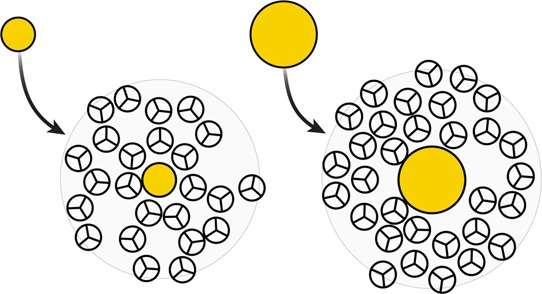
A small solute is compatible with water’s natural cages. A large solute does not fit in a cage. In cold water, small solutes can fit in the available cavities with minimal perturbation of the water structure. This process is favored by enthalpy and opposed by entropy. However, in cold water, big solutes do not fit in preexisting cavities. First-shell solvating water molecules around large nonpolar solutes are more disordered. Dissolving large solutes in cold water is opposed by enthalpy (breaks hydrogen bonds) and favored by entropy.
