ABSTRACT
Petroleum wastewater samples from oil refinery and oil exploration site were treated by hyper phenol-tolerant Bacillus cereus (AKG1 and AKG2) in laboratory-scale batch process to assess their bioremediation efficacy. Quality of the treated wastewater samples were analyzed in terms of removal of chemical oxygen demand (COD), total organic carbon (TOC) and ammonium nitrogen content, and improvement of biological oxygen demand (BOD). Adaptation of these bacteria to the toxic environment through structural changes in their cell membranes was also highlighted. Among different combinations, the co-culture of AKG1 and AKG2 showed the best performance in degrading the wastewater samples.
KEYWORDS: Bacillus cereus, biodegradation, biological oxygen demand (BOD), chemical oxygen demand (COD), petroleum wastewater
Fossil fuel, is a major source of energy and will remain so in coming decades. However, environmental pollution due to production and transportation of refinery products poses critical challenges. Wastewater from petroleum refineries usually contains high concentrations of aliphatic and aromatic petroleum hydrocarbons which can easily contaminate sources of drinking water, downstream from discharges.1-3 Bioremediation of petroleum wastewater offers a potential option to address the environmental issues. Biodegradation behavior of individual components of petroleum wastewater such as phenol, benzene, hexadecane, xylene and toluene is well documented.3-5 However, bioremediation of real petroleum wastewater is complicated because it consists of numerous components with varying chemical structure and toxicity.
In this regard, we have reported, the isolation of Bacillus cereus (AKG1 MTCC9817 and AKG2 MTCC9818) showing high phenol tolerance and also studied their degradation pathways.6,7 Capabilities of these strains for degrading phenol, following their immobilization in calcium-alginate beads, have also been reported.8,9 Herein, we try to evaluate the capabilities of these bacteria (either as free cells or immobilized in calcium-alginate beads) in bioremediation of petroleum wastewater samples collected from oil refinery site (Sample I) and oil exploration site (Sample II). The bacterial strains were used in 5 different ways –free cells of AKG1, free cells of AKG2, free cells of co-cultured AKG1 and AKG2, immobilized AKG1 and immobilized AKG2 – for the biodegradation of wastewater samples over a period of 20 d.
Chemical oxygen demand or COD indirectly indicates the amount of organic pollutants present in a wastewater sample. Thus, it is an important parameter for any wastewater treatment process. The removal of COD (Table 1), when all combinations of free bacteria without external N2 source were considered, was found to be in the range of 34%- 83%, using external N2 source resulted in slight increase in COD removal. Overall, co-culture system showed the highest reduction in COD level irrespective of N2 source. Interestingly, COD removal by immobilized cells were lower than that of the free cells possibly due to mass transfer limitations.9 Phenolics are major organic compounds found in refinery wastewater and have a direct relation with COD. The biodegradation of wastewater samples in present studies was also observed to reduce the concentration of phenolics by 57% (Fig. 1) and 45% in Sample I and Sample II, respectively. The reduction in total organic carbon (TOC) present in wastewater samples (Table 1) also followed similar trend as that of COD. Without any external N2 source, Removal of TOC was found to be in the range of 30%- 83%. The TOC level was reduced by 45–84% and 35–77% in presence of organic and inorganic N2 sources, respectively.
Table 1.
Removal of COD and TOC from wastewater.
| COD removal (%) | ||||||
|---|---|---|---|---|---|---|
| Without supplement |
Organic nitrogen supplement (Yeast) |
Inorganic nitrogen supplement (Ammonium Sulfate) |
||||
| Sample I | Sample II | Sample I | Sample II | Sample I | Sample II | |
| Free AKG1 | 61.11 | 34.14 | 69.44 | 51.21 | 66.66 | 41.46 |
| Free AKG2 | 41.66 | 61.53 | 55.55 | 69.23 | 47.22 | 64.10 |
| Co-culture | 83.78 | 70 | 89.18 | 79.5 | 86.48 | 72.5 |
| Immobilized AKG1 | 24.32 | 10.25 | 24.12 | 10.02 | 24.40 | 10.25 |
| Immobilized AKG2 | 10.81 | 10.25 | 10.50 | 10.33 | 10.10 | 10.75 |
| TOC removal (%) | ||||||
| Free AKG1 | 57.12 | 30.00 | 65.45 | 48.00 | 63.51 | 35.00 |
| Free AKG2 | 34.68 | 41.59 | 45.52 | 55.99 | 39.77 | 45.00 |
| Co-culture | 83.45 | 54.00 | 84.54 | 75.95 | 77.28 | 70.00 |
| Immobilized AKG1 | 18.75 | 13.30 | — | — | — | — |
| Immobilized AKG2 | 8.29 | 11.80 | — | — | — | — |
Figure 1.
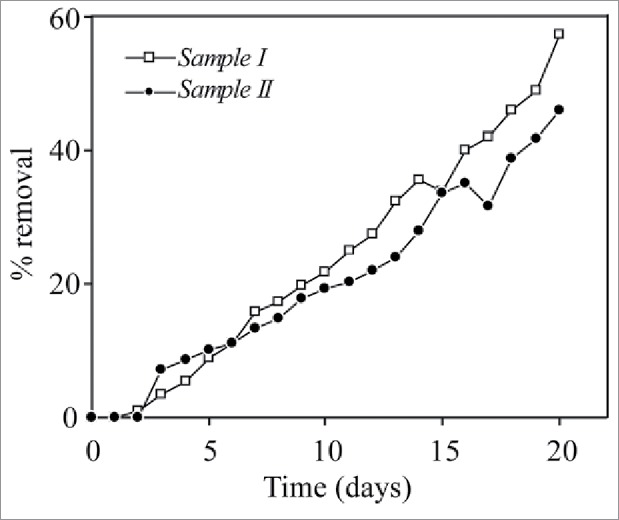
Removal of phenolics (%) from wastewater samples by co-culture system.
During microbial treatment of petroleum wastewater samples, in addition to COD removal, prominent increase in BOD was also observed (Figs. 2C and 2D). After 20 d of bioremediation, initial BOD values of 180 mg/L (sample I) and 420 mg/L (sample II) increased to as high as 861 mg/L and 1532 mg/L, respectively. The ratio of BOD to COD is an index of biodegradability performance. The low BOD/COD ratio (0.030∼0.065) of wastewater samples before bioremediation indicated difficulty of treating them by biological methods. However, after treatment, the ratio was increased to 0.89 and 0.79 for sample I and sample II, indicating significant improvement of biodegradability of the samples. BOD/COD ratio of 0.9∼1.0 is generally considered as the cut-off point for the biodegradable wastewater.10,11 Excess N2 of wastewater should be removed as it may lead to eutrophication and toxicity.12 During bioremediation of wastewater, this is achieved by biological nitrification. A satisfactory level of NH4+-N removal from wastewater samples (Table 2) was achieved by co-culture system (Sample I: 68.12% and Sample II: 69.45%) followed by strain AKG1 (54%) for sample I and AKG2 (82.46%) for sample II. In case of the immobilized system, the reduction varied in the range of 31.5% ∼42.46% by the various strains.
Figure 2.
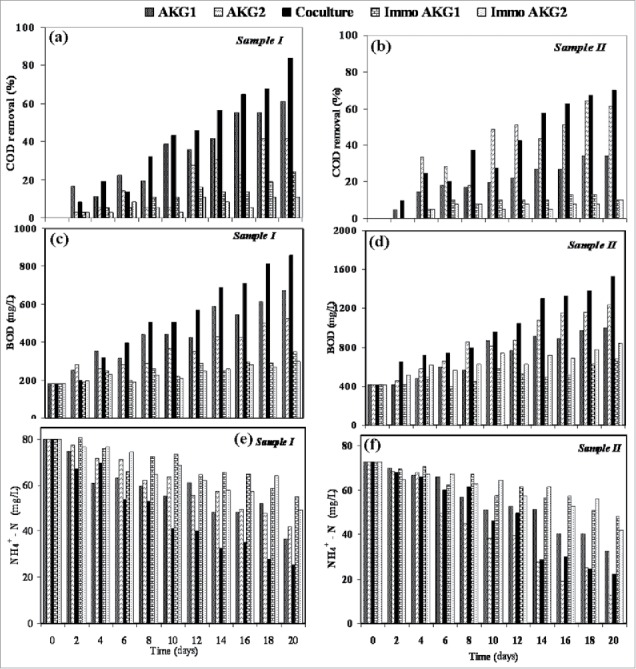
Effect of bioremediation on COD removal (A and B), BOD increment (C and D) and ammonia-nitrogen removal (E and F).
Table 2.
Removal of Ammonium- nitrogen from wastewater.
| % of Ammonia-Nitrogen reduction after 20 days |
|||||
|---|---|---|---|---|---|
| Sample | AKG1 | AKG2 | Co-culture | Immobilized AKG1 | Immobilized AKG2 |
| Sample I | 54 | 47.5 | 68.12 | 31.5 | 38.5 |
| Sample II | 55.34 | 82.46 | 69.45 | 33.83 | 42.46 |
Microorganisms are able to tolerate several toxic organic compounds by by means of different kinds of adaptive mechanisms including changes in the fatty acid composition of membrane lipids.13-16 Fourier transform infrared (FTIR) spectra of cell membranes isolated from control bacteria and those employed in bioremediation of wastewater samples showed several absorption peaks indicating various functional groups (Figure 3). Close inspection of the absorption peaks of wastewater exposed bacteria showed shift of bands relative to those observed in case of phenol used as substrate (Fig. 3B and 3D). These shifts in FTIR suggested that polysaccharides and proteins (major components of membrane) were affected and evolved their structure in the presence of wastewater.17 Overall, co-culture of the free cells of B. cereus AKG1 and AKG2 showed the best performance in bioremediation of petroleum wastewater samples in the present study.
Figure 3.
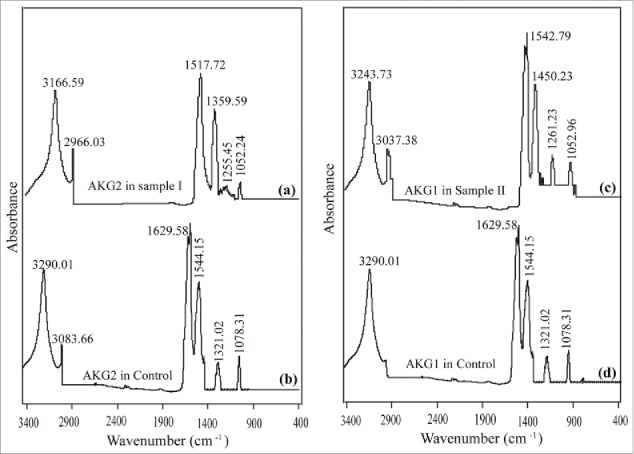
FTIR of bacterial membrane isolated from bacteria grown in control (phenol) and wastewater samples.
Experimental
Strains AKG1 and AKG26 were grown in 1 g/L phenol-MSM medium as reported earlier.6-9 100 mL of sterilized wastewater samples were taken into conical flasks and then incubated with specific strain (OD = 0.8) at their optimum growth condition (37°C at 120 rpm with definite pH condition) for desired period of time. Immobilized bacteria employed for bioremediation were prepared by previous method.8,9 During the experiment, samples (2 mL) were withdrawn time -to- time to measure COD and NH4+-N by previous method.16 TOC content of the samples was determined in a TOC analyzer (O.I. analytical Aurora, Model 1030). Concentrations of phenolic compounds were analyzed by high performance liquid chromatography (HPLC, ProSTAR, Varian) equipped with a UV–visible detector and C18 column.
In order to study bacterial membrane adaptation, cells were grown in MSM medium with either phenol (control group) or wastewater as the carbon source. Grown bacteria were harvested by centrifugation (8000 rpm, 10 min) and bacterial membrane was isolated by previously reported method.18 Isolated membranes were lyophilized for further FT-IR analysis in Perkin-Elmer, PE-RXI.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
This work was supported by the Council of Scientific and Industrial Research (CSIR) (24(0302)/09/EMR-II, Dated: 04–03–2009), New Delhi, Government of India, India.
References
- [1].Hoffman DJ, Rattner BA, Burton GA Jr, Cairns J Jr. Handbook of ecotoxicology. CRC Press, 2002. [Google Scholar]
- [2].Hu G, Li J, Zeng G. Recent development in the treatment of oily sludge from petroleum industry: A review. J Hazard Mater 2013; 261:470-90; PMID:23978722; http://dx.doi.org/ 10.1016/j.jhazmat.2013.07.069 [DOI] [PubMed] [Google Scholar]
- [3].Singleton I. Microbial metabolism of xenobiotics: fundamental and applied research. J Chem Technol Biotechnol 1994; 59:9-23; http://dx.doi.org/ 10.1002/jctb.280590104 [DOI] [Google Scholar]
- [4].Organization WH Phenol, environmental health criteria-EHC 161. WHO, Geneva: 1994. [Google Scholar]
- [5].Mahajan S. Pollution control in process industries. Tata McGraw-Hill Education, 1985. [Google Scholar]
- [6].Banerjee A, Ghoshal AK. Isolation and characterization of hyper phenol tolerant Bacillus sp. from oil refinery and exploration sites. J Hazard Mater 2010; 176:85-91; http://dx.doi.org/ 10.1016/j.jhazmat.2009.11.002 [DOI] [PubMed] [Google Scholar]
- [7].Banerjee A, Ghoshal AK. Phenol degradation by Bacillus cereus: Pathway and kinetic modeling. Bioresource Technol 2010; 101:5501-7; http://dx.doi.org/ 10.1016/j.biortech.2010.02.018 [DOI] [PubMed] [Google Scholar]
- [8].Banerjee A, Ghoshal AK. Phenol degradation performance by isolated Bacillus cereus immobilized in alginate. Inter Biodeterioration Biodegradation 2011; 65:1052-60; http://dx.doi.org/ 10.1016/j.ibiod.2011.04.011 [DOI] [Google Scholar]
- [9].Banerjee A, Ghoshal AK. Biodegradation of phenol by calcium-alginate immobilized@@ Bacillus cereus in a packed bed reactor and determination of the mass transfer correlation. J Environ Chem Engineer 2016; 4:1523-9; http://dx.doi.org/ 10.1016/j.jece.2016.02.012 [DOI] [Google Scholar]
- [10].Celenza G. Industrial Waste Treatment Process Engineering: Biological Processes. CRC Press, 1999. [Google Scholar]
- [11].Contreras S, Rodrıguez M, Momani FA, Sans C, Esplugas S. Contribution of the ozonation pre-treatment to the biodegradation of aqueous solutions of 2, 4-dichlorophenol. Water Res 2003; 37:3164-71; PMID:14509703; http://dx.doi.org/ 10.1016/S0043-1354(03)00167-2 [DOI] [PubMed] [Google Scholar]
- [12].Sarioglu M. Removal of ammonium from municipal wastewater using natural Turkish (Dogantepe) zeolite. Separ Purific Technol 2005; 41:1-11; http://dx.doi.org/ 10.1016/j.seppur.2004.03.008 [DOI] [Google Scholar]
- [13].Ramos JL, Duque E, Rodríguez-Herva JJ, Godoy P, Haïdour A, Reyes F, et al.. Mechanisms for solvent tolerance in bacteria. J Biol Chem 1997; 272:3887-90; PMID:9020089; http://dx.doi.org/ 10.1074/jbc.272.7.3887 [DOI] [PubMed] [Google Scholar]
- [14].Gao B, Zhu X, Xu C, Yue Q, Li W, Wei J. Influence of extracellular polymeric substances on microbial activity and cell hydrophobicity in biofilms. J Chem Technol Biotechnol 2008; 83:227-32; http://dx.doi.org/ 10.1002/jctb.1792 [DOI] [Google Scholar]
- [15].Yu GH, He PJ, Shao LM, Zhu YS. Extracellular proteins, polysaccharides and enzymes impact on sludge aerobic digestion after ultrasonic pretreatment. Water Res 2008; 42:1925-34; PMID:18082240; http://dx.doi.org/ 10.1016/j.watres.2007.11.022 [DOI] [PubMed] [Google Scholar]
- [16].Liu Y, Yang CH, Li J. Adhesion and retention of a bacterial phytopathogen Erwinia chrysanthemi in biofilm-coated porous media. Environ Sci Technol 2007; 42:159-65; http://dx.doi.org/ 10.1021/es071698k [DOI] [PubMed] [Google Scholar]
- [17].Long G, Zhu P, Shen Y, Tong M. Influence of extracellular polymeric substances (EPS) on deposition kinetics of bacteria. Environ Sci Technol 2009; 43:2308-14; PMID:19452879; http://dx.doi.org/ 10.1021/es802464v [DOI] [PubMed] [Google Scholar]
- [18].Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Canadian J Biochem Physiol 1959; 37:911-7; PMID:13671378; http://dx.doi.org/ 10.1139/o59-099 [DOI] [PubMed] [Google Scholar]


