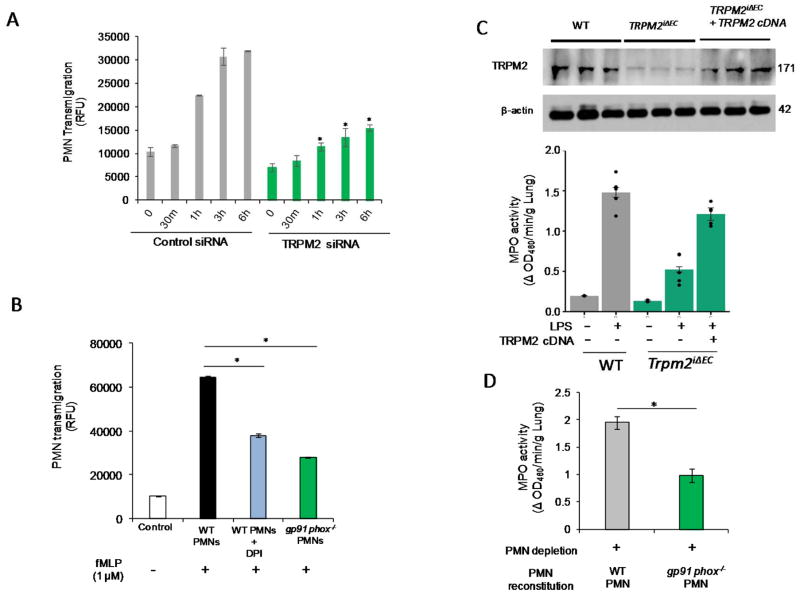
Figure 6. Endothelial cell expressed TRPM2 is responsible for PMN transmigration whereas expression of TRPM2 in TRPM2iΔEC mice restores defective PMN uptake phenotype.
(A) Time dependent transmigration of fMLP (1μM)-activated PMNs in control and TRPM2-siRNA treated HLMVECs measured as described in Methods. TRPM2 silencing in endothelial cells suppressed PMN transmigration. (B) Transmigration of untreated WT PMNs, DPI treated PMNs or gp91phox−/− PMNs across mouse endothelial cells at 6h post-fMLP stimulation. PMNs treated with DPI or PMNs from gp91phox−/− mice showed defective transmigration across mouse endothelial cells. (C) TRPM2 overexpression in Trpm2iΔEC mice using liposomes restored the defective PMN uptake phenotype of Trpm2iΔEC mice. (D) PMNs in WT mice were depleted using Gr-1 antibody (i.p. 100 μg/mice) and after 24h of i.p. antibody injection, mice were reconstituted with gp91phox−/− PMNs and were simultaneously injected i.p. LPS for 24h and were then assayed for MPO activity in lungs. Mice reconstituted with gp91phox−/− PMNs showed defective PMN uptake in lungs compared to the WT PMNs reconstituted mice. *p<0.05 compared to the respective control group.