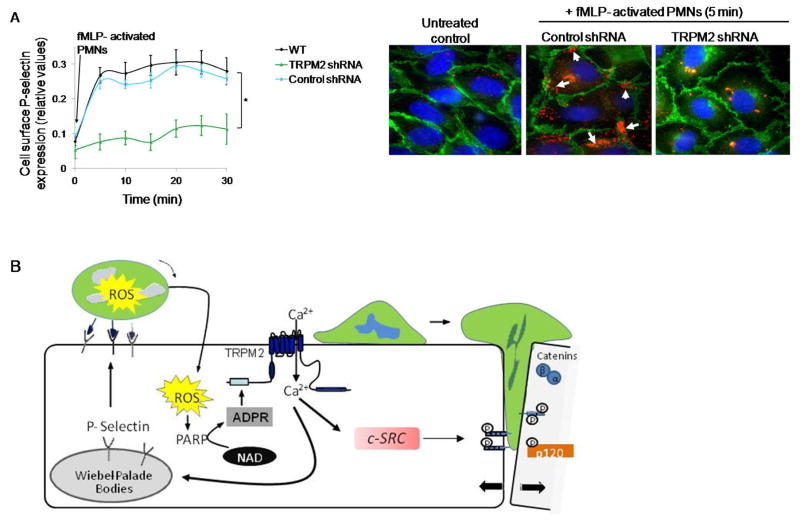
Figure 7. Activated PMN-dependent P-selectin translocation to endothelial cell surface requires TRPM2.
(A) HLMVECs transduced with shRNA to silence TRPM2 expression were incubated with fMLP (1μM)-activated PMNs (WT, 1 EC:5 PMN ratio) for varying times. Cell surface expression of P-selectin was determined by enzyme linked immunosorbent assay (ELISA) using tetramethyl-benzidine substrate (results are shown as mean ± SEM; n= 3). (B) Endothelial monolayers layered with fMLP (1μM) -activated PMNs for 5 min, and then fixed and stained with Alexa 594 mouse IgG/Pselectin (red), Alexa-488 goat IgG/VE-cadherin (green), and DAPI (blue). Scale bar=20 μM. Each image is representative of three experiments. Control endothelial cells expressed little surface P-selectin whereas PMN activation increased P-selectin-expression along cell borders (arrows). Depletion of TRPM2 prevented PMN-elicited cell surface redistribution of P-selectin. (C) Model derived from our results. PMN interaction with ECs delivers a ROS signal which activates TRPM2-dependent gating of Ca2+ via the generation of ADPR through the catalytic activity of PARP-1. This in turn results in activation of c-Src and phosphorylation of VE-cadherin through intermediate steps, the disassembly of AJs and transmigration of PMNs. Another crucial TRPM2 Ca2+ gating step is the Ca2+ signaling dependent release of P-selectin from Weibel Palade bodies, which induces EC surface localization of P-selectin to promote PMN arrest on EC surface and thus may fine tune PMN interaction with ECs to amplify PMN dependent TRPM2 activation. *p<0.05 compared to the respective control group.