Figure 5. Intermediary metabolic pathways in prolonged fasting.
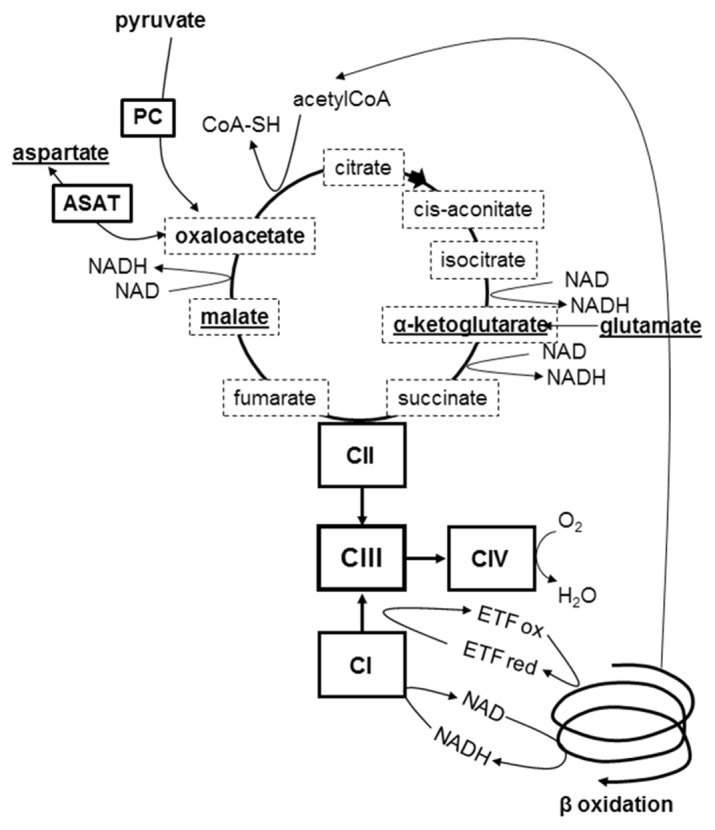
Main substrates of gluconeogenesis are show in bold, those involved in the malate-aspartate shuttle are underlined; Krebs intermediates are framed with a dotted line; PC= pyruvate carboxylase; ASAT= aspartate aminotransferase; CI= respiratory complex I, CII= complex II, CIII= complex III, CIV= complex IV; ETF ox and ETF red= electron transfer flavoprotein oxidized and reduced state. During prolonged fasting a defective complex III is bound to be overloaded by the convergent increased electrons fluxes from activated gluconeogenesis and β oxidation. Redox potential increases, resulting in decreased flux in the Krebs cycle oxidoreduction steps. Defect in gluconeogenic substrates induces hypoglycemia, defect in aspartate essential for argininosuccinate synthesis induces hyperammonemia and insufficient entry of acetylCoA into Krebs cycle induces ketosis. Graphical abstract
