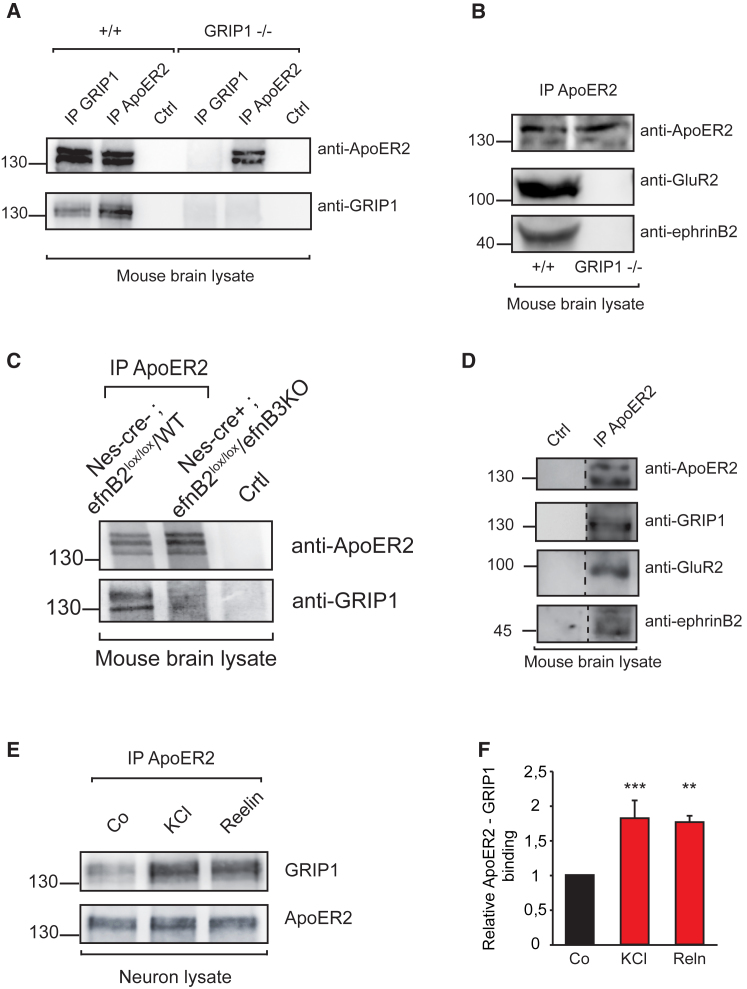
Figure 3.
GRIP1 Binds to ApoER2 and Scaffolds an EphrinB2/ApoER2/AMPA Receptor Complex
(A) GRIP1 and ApoER2 co-immunoprecipitate in mouse brain lysates. Total brain lysates from adult wild-type (+/+) and GRIP1 knockout (GRIP−/−) mice were immunoprecipitated with anti-GRIP1, anti-ApoER2, or an unrelated antibody generated in rabbit (Ctrl) and analyzed by western blot for GRIP1 and ApoER2.
(B) GRIP1 bridges ApoER2, GluR2, and ephrinB2. Wild-type (+/+) and GRIP1 knockout (GRIP1−/−) brain lysates were immunoprecipitated using anti-ApoER2 antibody, and binding to GluR2 and ephrinB2 was analyzed by western blot.
(C) EphrinB ligands mediate the interaction between ApoER2 and GRIP1. Brain lysates from adult control (Nes-cre−; efnB2lox/lox/efnB3+/+) and double ephrinB2/ephrinB3 knockout (Nes-cre+; efnB2lox/lox/efnB3−/−) mice were immunoprecipitated with anti-ApoER2 antibody or normal rabbit immunoglobulin G (IgG; Ctrl) and analyzed by western blot using anti-GRIP1 antibody.
(D) Co-immunoprecipitation using anti-ApoER2 antibody or an unrelated rabbit antibody (Ctrl) shows the macromolecular complex formed by ApoER2, GRIP1, GluR2, and ephrinB2.
(E and F) Interaction between ApoER2 and GRIP1 is increased upon stimulation with KCl or Reelin in primary hippocampal neuron cultures. Western blots showing GRIP1 co-immunoprecipitation by using an anti-ApoER2 antibody (E). Quantification of relative binding of GRIP1 to ApoER2 upon KCl or Reelin stimulation is shown (n = 3) (F).
Bar graphs show mean ± SEM (shown as error bars).∗∗p < 0.01, ∗∗∗p < 0.001.