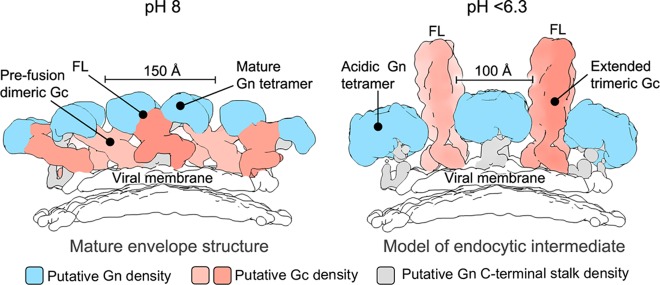
FIG 5.
A model of pH-dependent conformational changes to the Gn and Gc glycoproteins during endocytosis, prior to fusion. At left is a side-view schematic of the mature hantavirus envelope, based upon a previously reported reconstruction of the TULV glycoprotein spike (EMDB accession no. EMD-3364). The globular Gn glycoprotein (blue), Gc glycoprotein (salmon), C terminus of the Gn-glycoprotein (gray), and putative location of the Gc fusion loops (FL; buried in the Gn-Gc interface), are indicated. Disassembly of the Gn-Gc spike complex (right panel) and the formation of the acidic Gn tetramer could create the space necessary for trimerization of the Gc glycoprotein with the fusion loops facing outwards to enable contact with target cellular membranes. The Gc trimer shown in the schematic is based on the HTNV Gc crystal structure (PDB accession number 5LJZ) (14), with domain III rotated 90° to model an extended class II fusion protein trimer that has been suggested to exist at low pH, prior to membrane fusion (35, 39). The width of the putative Gn tetramers formed under both pH-neutral and acidic conditions is annotated.