Abstract
Cellular prion protein (PrPC), the infective agent of transmissible spongiform encephalopathies, is thought to be related to several cellular physiological and physiopathological processes. We have previously reported that PrPC participates in multi-drug-resistance of gastric cancer. As the salient ligand molecule of PrP for participating in internalization and propagation of the scrapie form of prion protein (PrPSc), 37 kDa laminin receptor precursor protein (37LRP) shared the same gene coding sequence of MGr1-Ag, another protein previously found to be involved in multi-drug-resistance of gastric cancer in our lab. In the present study, we explored whether MGr1-Ag/37LRP contributed to PrPC mediated multi-drug-resistance in gastric cancer. Immunohistochemical staining showed similar expression patterns of MGr1-Ag/37LRP and PrPC in gastric cancer tissue serial sections. Western blot and immunohistochemistry also demonstrated correlative expression of MGr1-Ag/37LRP and PrPC in gastric cancer cell lines. Interaction between MGr1-Ag/37LRP and PrPC in gastric cancer cell lines and gastric cancer tissues were verified by immunofluorescence and co-immunoprecipitation. Furthermore, knockdown of MGr1-Ag/37LRP significantly attenuated PrPC induced multi-drug-resistance by sensitizing drug-induced apoptosis through inhibition of AKT activation. In conclusion, MGr1-Ag/37LRP may interact with PrPC and promote the PrPC induced multi-drug-resistance in gastric cancer through PI3K/AKT pathway. The current study elucidates the mechanism of how PrPC triggers intracellular signaling cascade resulting in multi-drug-resistance phenotype and provides a novel candidate molecular target against gastric cancer.
Keywords: PrPC, MGr1-Ag/37LRP, gastric cancer, multi-drug-resistance (MDR), apoptosis
INTRODUCTION
PrPC is the cellular form of prion protein, which is generally considered to be the infective agent of transmissible spongiform encephalopathies both in human and animals [1]. As a glycosyl-phosphatidylinositol-anchored membrane bound glycoprotein, PrPC is ubiquitously expressed in mammalian cells especially in neurons and is highly conserved among species [2]. However, the physiological role of PrPC still remains elusive. To date, the putative roles of PrPC are thought to be related to a lot of physiological and physiopathologic processes including cell adhesion, cell growth and proliferation, cell death, signal transduction, as well as cholesterol, iron, zinc and copper metabolism [3–7]. Our previously studies showed that PrPC is highly expressed in gastric cancer tissues and gastric cancer cell lines and has notable effects on tumorigenesis and metastasis of gastric cancer [8, 9]. Moreover, we also previously reported that PrPC is related to multi-drug-resistance with aberrant high expression in Adriamycin(ADR)-resistant gastric adenocarcinoma cell line SGC7901/ADR and Vincristine(VCR)-resistant cell line SGC7901/VCR, [10] which also displayed cross-resistance to other anticancer drugs [11]. Previous data demonstrated that ectopic over-expression of PrPC might induce gastric cancer cells to display significantly enhanced resis tance to chemical therapeutic drugs [12].
To gain a better insight into prion's putative role, studies looking for molecules interacting with prion protein have identified divalent metal ions, several proteins, and nucleic acids [13] Among them, the 37KDa laminin receptor precursor (37LRP) was thought to be a salient ligand of PrP, [14] which plays an important role in internalization, propagation of the PrPSc and scrapie desease infection [15–17]. 37LRP shares the same gene coding sequence with MGr1-antigen (MGr1-Ag), a novel protein we have found contributes to multi-drug-resistance of gastric cancer [18]. Hypoxia-mediated up-regulation of MGr1-Ag/37LRP occurs via a HIF1-dependent mechanism and contributes to drug resistance in gastric cancers [19].
Since we found that PrPC could promote drug resistance in gastric cancer, questions about how PrPC triggers the intracellular signaling cascade remain. For example, whether there is a binding partner that functions synergistically with PrPC and contributes to the multi-drug-resistance phenotype is unknown. We found that PrPC and MGr1-Ag/37LRP are involved in hypoxia-mediated gastric cancer MDR. Moreover, they were verified to alter the tumorous drug resistant phenotypes through similar pathways [12, 20]. Thus, we presume that MGr1-Ag/37LRP probably is important in the mechanism of PrPC related multi-drug-resistance in gastric cancer. In the present study, we explored whether MGr1-Ag/37LRP contributes to PrPCmediated multi-drug-resistance, which may provide an understanding of the specific mechanism of PrPC-mediated multi-drug-resistance.
RESULTS
MGr1-Ag/37LRP and PrPC are correlatively expressed in gastric cancer tissues and gastric cancer cell lines
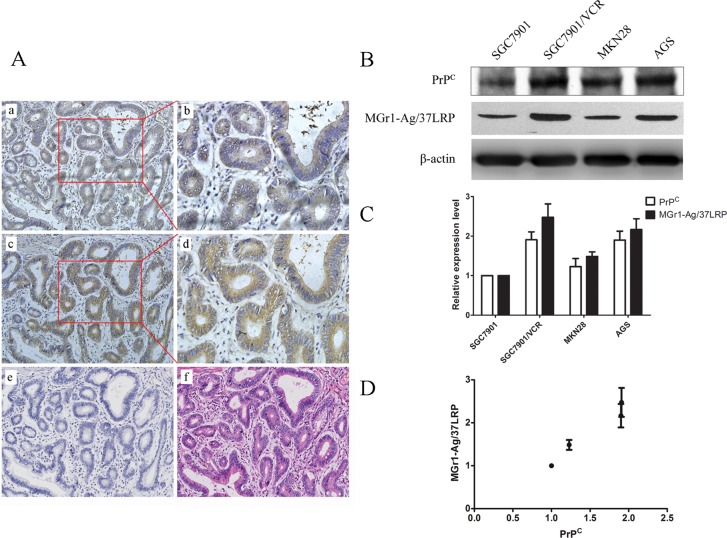
Immunohistochemical staining showed a similar expression pattern of MGr1-Ag/37LRP and PrPC in gastric cancer tissue serial sections (Figure 1A). MGr1-Ag/37LRP could be detected positively in 52% of the 50 patients, while PrPC could be detected positively in 66% of the patients. Paired immunostaining analysis revealed a strong correlation between MGr1-Ag/37LRP and PrPC expression (Table 1, p < 0.05). Furthermore, western blot analysis showed a significant correlative expression (p < 0.05) between MGr1-Ag/37LRP and PrPC in gastric cancer cell lines SGC7901, SGC7901/VCR, MKN28 and AGS (Figure 1B–1D). The expression levels of PrPC and MGr1-Ag/37LRP protein were both significantly higher (p < 0.05) in SGC7901/VCR than in SGC7901 cells (Figure 1B and 1C), indicating they may both promote the drug resistance phenotype of gastric cancer.
Figure 1. Expression pattern of PrPC and MGr1-Ag/37LRP protein in gastric cancer tissue and cell lines.
(A) Results from immunohistochemical staining of MGr1-Ag/37LRP and PrPC in gastric cancer tissue serial sections. Upper row: expression pattern of MGr1-Ag/37LRP in gastric cancer tissue (a 200×, b 400×); middle row: expression pattern of PrPC in gastric cancer tissue (c 200×, d 400×); lower row: negative control (e 200×) and HE staining (f 200×). (B–D) Western blot analysis of PrPC and MGr1-Ag/37LRP protein expression level in gastric cell lines SGC7901, SGC7901/VCR, MKN28 and AGS, β-actin was used as internal control.
Table 1. Expression of MGr1-Ag/37LRP andPrPC in gastric cancer tissue.
| MGr1-Ag/37LRP (%) | Total | |||
|---|---|---|---|---|
| + | – | |||
| PrPC (%) | + | 22 (44%) | 11 (22%) | 33(66%) |
| – | 4 (8%) | 13 (26%) | 17(34%) | |
| Total | 26 (52%) | 24(48%) | 50 | |
χ2 test, P < 0.05.
Co-localization of MGr1-Ag/37LRP and PrPC in gastric cancer cell lines and gastric cancer tissues
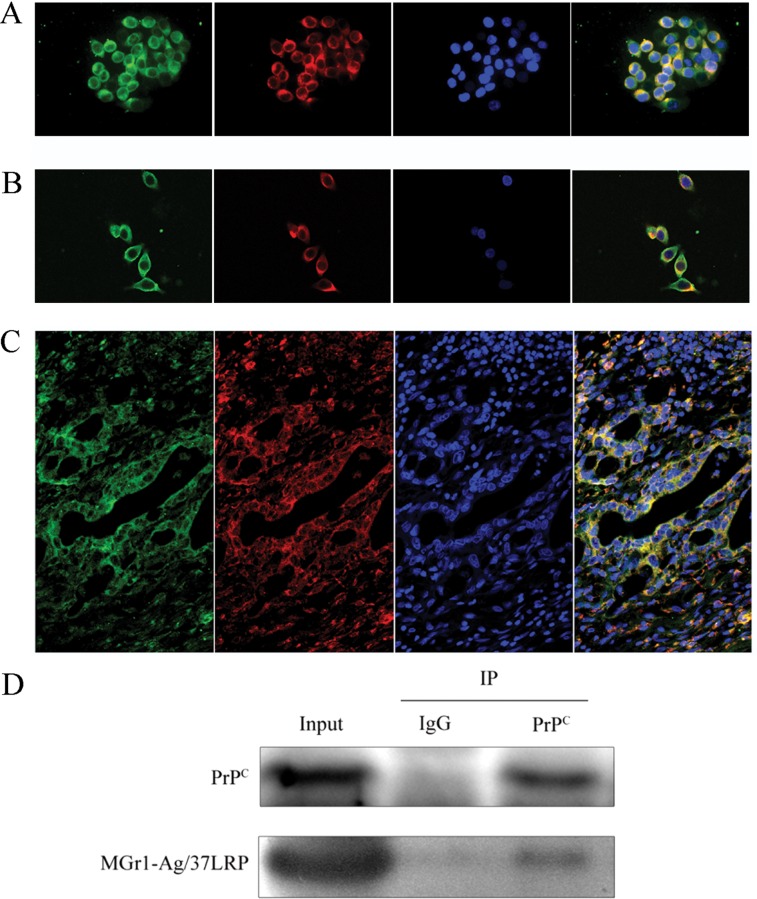
Furthermore, co-localization of PrPC and MGr1-Ag/37LRP protein in gastric cancer cells and tissues was tested by immunofluorescence analysis using laser scanning confocal fluorescence assay. As shown in Figure 2A, MGr1-Ag/37LRP and PrPC protein were co-localized in the cytoplasm and partially on the memberane but rare in nucleus of gastric cancer cell lines AGS and SGC7901 Figure 2A and 2B. We have noticed the abnormally high expression level of both MGr1-Ag/37LRP and PrPC in the cytoplasm of gastric cancer cells, which is consistent with our previous study and results of other researchers. Castronovo V et al. reported that the majority of 37LRP was located in the cytoplasm although it had some membrane-associated domains [21]. We hypothesized such subcellular localization changes facilitates the MGr1-Ag/37LRP and PrPC interaction with other intracellular proteins, which might be one of the mechanisms by which MGr1-Ag/37LRP and PrPC participate in gastric cancer multi-drug-resistance. Co-localization of MGr1-Ag/37LRP and PrPC protein was also observed in gastric cancer tissues glands (Figure 2C). We also confirmed the interaction between MGr1-Ag/37LRP and PrPC protein by a co-immunoprecipitation assay (Figure 2D). These results provided strong evidence that these two proteins coexist in a protein complex and interact in gastric cancer cells.
Figure 2. Co-localization of PrPC and MGr1-Ag/37LRP in gastric cancer cell lines and tissues.
(A) and (B) Co-localization of PrPC and MGr1-Ag/37LRP was examined by double immunofluorescence staining. Merged image (yellow) revealed co-localization between PrPC (red) and MGr1-Ag/37LRP(green) in gastric cancer cell lines AGS (A) and SGC7901 (B). The nucleus was identified with DAPI (blue). (C) Co-localization (yellow) of PrPC (red) and MGr1-Ag/37LRP (green) in gastric cancer tissues. (D) Interaction between PrPC and MGr1-Ag/37LRP in SGC7901/VCR cells was further tested by co-immunoprecipitation using anti-PrPC antibodies. Both PrPC and MGr1-Ag/37LRP could be detected by their antibodies respectively in anti-PrPC immunoprecipitate sample of SGC7901/VCR.
MGr1-Ag/37LRP protein expression might be regulated by PrPC in gastric cancer cells
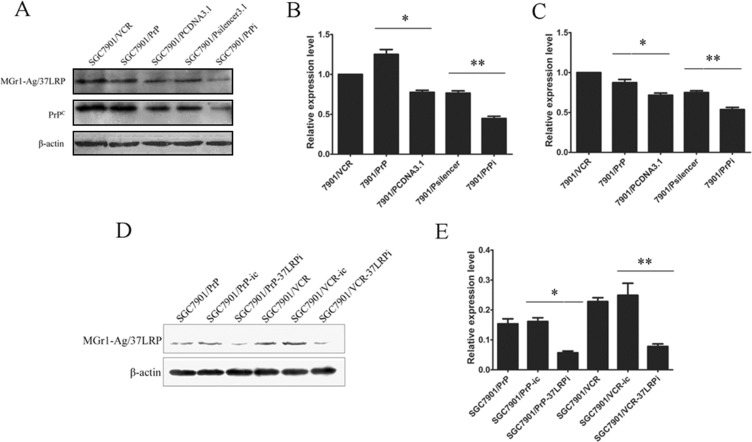
We established stable transfected cell lines SGC7901/PrP and SGC7901/PrPsi in our previous work [8]. So we detected whether MGr1-Ag/37LRP protein was altered in those PrP derived gastric cancer cell lines. Western blot image (Figure 3A) and grayscale analysis (Figure 3B and 3C) showed that the expression of MGr1-Ag/37LRP protein were significantly increased in SGC7901/PrP compared with SGC7901/PCDNA3.1 and decreased in SGC7901/PrPi compared with SGC7901/Psilencer3.1 (p < 0.05), revealing a regulation of protein expression of MGr1-Ag/37LRP by PrPC in gastric cancer cells.
Figure 3. The protein expression of MGr1-Ag/37LRP in PrPC up-regulated and down-regulated gastric cancer cells.
(A–C) Western blot analysis of MGr1-Ag/37LRP expression in PrPC up-regulated and down-regulated gastric cancer cells. PrPC expression in SGC7901/PrP (lane 2) increased significantly compared to SGC7901/PCDNA3.1 (lane 3) (*p < 0.05 in B) and decreased in SGC7901/PrPi (lane 4) compared to SGC7901/Psilencer3.1 (lane 5) (**p < 0.05 in B). MGr1-Ag/37LRP protein expression was also higher in SGC7901/PrP and lower in SGC7901/PrPi (A&C). SGC7901/VCR was used as positive control and β-actin as internal control. (D) and (E) Western blot analysis showed that expression level of MGr1-Ag/37LRP protein was markedly suppressed in SGC7901/PrP (lane 3) and SGC7901/VCR (lane 6) compared with siRNA negative control (lane 2 and lane 4) after transiently transfection of MGr1-Ag/37LRP siRNA (**p < 0.05 in E). β-actin was used as internal control.
Influence of MGr1-Ag/37LRP on drug sensitivity of PrPC derived gastric cancer cell lines in vitro
Since both MGr1-Ag/37LRP and PrPC have been reported to be involved in multi-drug-resistance of gastric cancer, we explored whether MGr1-Ag/37LRP could influence the drug resistance activity of PrPC. SiRNA targeting MGr1-Ag/37LRP was employed as a tool for interfering with the expression of MGr1-Ag/37LRP. Western blot analysis (Figure 3D and 3E) showed that compared with cells transfected with control oligonucleotides, MGr1-Ag/37LRP protein expression was significantly decreased in the cells transiently transfected with MGr1-Ag/37LRP siRNA (p < 0.05). In vitro effects of the drugs on the growth of SGC7901/PrP-37LRPi compared with SGC7901/PrP-ic, and SGC7901/VCR-37LRPi compared with SGC7901/VCR-ic were evaluated using MTT assay respectively. As reported in Table 2, knockdown of MGr1-Ag/37LRP by siRNA could notably increase the drug sensitivity of SGC7901/PrP and SGC7901/VCR to P-gp related drugs ADR, VCR and P-gp non-related drugs 5-FU and CDDP (p < 0.05). SGC7901 cells were employed as a positive control and showed significantly decreased IC50 values to drugs compared to SGC7901/PrP and SGC7901/VCR (p < 0.05). These data suggested that MGr1-Ag/37LRP might contribute to PrPC-induced multi-drug-resistant phenotypes in gastric cancer cells possibly not through P-gp related pathways.
Table 2. MGr1-Ag/37LRP siRNA attenuates multi-drug-resistance in PrPC derived gastric cancer cells (IC50 of drugs).
| Cell lines | Treatment | 5-FU | CDDP | ADR | VCR | |
|---|---|---|---|---|---|---|
| 7LRP siRNA | siRNA NC | |||||
| SGC7901/PrP | – | + | 0.13 ± 0.01 | 1.88 ± 0.26 | 1.57 ± 0.18 | 7.49 ± 1.27 |
| + | – | 0.06 ± 0.01† | 0.19 ± 0.06† | 0.84 ± 0.11† | 4.41 ± 0.19† | |
| SGC7901/VCR | – | + | 1.88 ± 0.57 | 5.27 ± 0.54 | 3.77 ± 0.46 | 17.42 ± 2.09 |
| + | – | 0.55 ± 0.10‡ | 1.63 ± 0.28‡ | 1.94 ± 0.27‡ | 7.53 ± 0.51‡ | |
†p < 0.05 vs. SGC7901/PrP cells transfected with siRNA negative control.
‡p < 0.05 vs. SGC7901/VCR cells transfected with siRNA negative control.
MGr1-Ag/37LRP contributes to PrPC mediated gastric cancer multi-drug-resistance through altering resistance ability of gastric cancer cells to drug-induced apoptosis
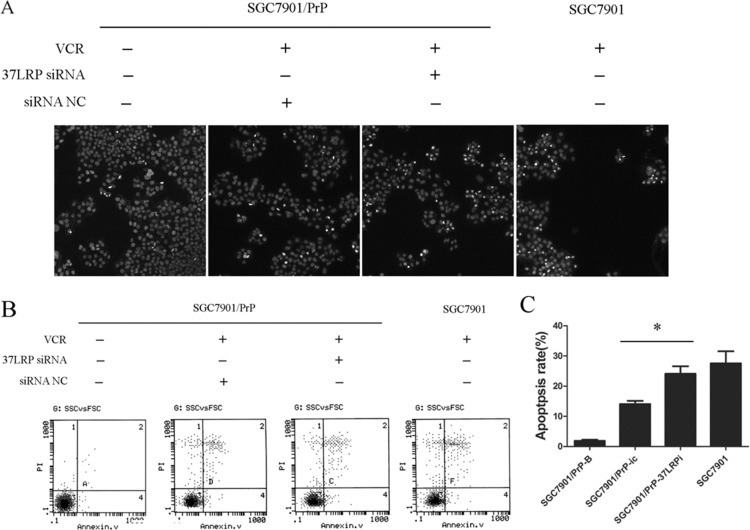
The suppression of drug-induced apoptosis is an important mechanism in multi-drug-resistance of cancer. We examined the effect of MGr1-Ag/37LRP on VCR-induced apoptosis in SGC7901-derived cells. Hoechst staining showed that apoptotic cells were significantly increased in SGC7901/PrP-37LRPi compared with SGC7901/PrP-ic after treatment with VCR (Figure 4A). Annexin V/PI double staining showed that apoptosis rate of SGC7901/PrP-37LRPi was much higher than that of control SGC7901/PrP-ic cells after same dose treatment of VCR (Figure 4B and 4C, p < 0.05). SGC7901 cells (positive control) also showed a higher apoptotic rate compared with SGC7901/PrP-ic (p < 0.05) but had no significant difference with SGC7901/PrP-37LRPi cells (p > 0.05). These results revealed that PrP contributes to a more severe resistance to VCR-induced apoptosis and synergistically functions with MGr1-Ag/37LRP in gastric cancer.
Figure 4. MGr1-Ag/37LRP contributes to PrPC mediated gastric cancer multi-drug-resistance through promoting apoptosis.
(A) Hoechst staining results of SGC7901/PrP cells with or without MGr1-Ag/37LRP siRNA to VCR inducted apoptosis. Apoptotic cells were significantly increased in SGC7901/PrP-37LRPi compared with SGC7901/PrP-ic after treated with VCR. SGC7901 was employed as a positive control. SGC7901/PrP cells without VCR were used as a negative control. (B and C) Apoptosis rate of SGC7901/PrP cells with or without MGr1-Ag/37LRP siRNA to VCR was tested by flow cytometry. Apoptosis rate of SGC7901/PrP-37LRPi was much higher than that of control SGC7901/PrP-ic cells under the same dose of VCR treatment (*p < 0.05). SGC7901 was employed as a positive control. SGC7901/PrP cells without VCR treatment were used as a negative control. SGC7901 cells (positive control) also showed a higher apoptotic rate compared with SGC7901/PrP-ic (p < 0.05) but had no significant difference with SGC7901/PrP-37LRPi cells (p > 0.05).
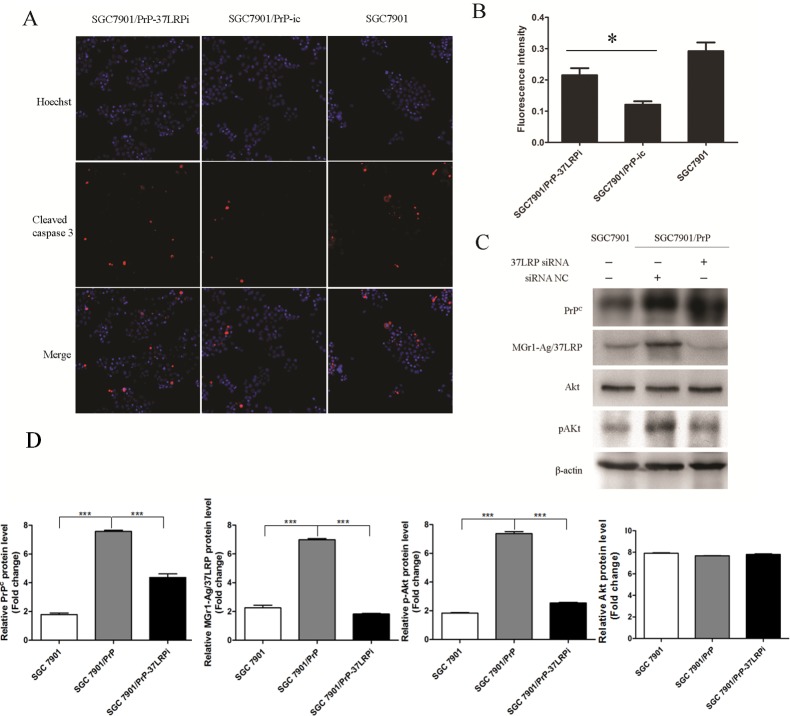
MGr1-Ag/37LRP promotes PrPC induced gastric cancer multi-drug-resistance by activation of Caspase 3 through PI3K/AKT pathway
MGr1-Ag/37LRP siRNA significantly increased caspase 3 activation in SGC7901/PrP compared with control group (Figure 5A and 5B, p < 0.05). PI3K/AKT signaling pathway is well-known to be critical in cell apoptosis. More importantly, PrPC was verified to promote proliferation and multi-drug-resistance of gastric cancer cells though activation of PI3K/Akt pathway in our previous work [22]. So we investigated whether the PI3K/AKT pathway was regulated by MGr1-Ag/37LRP in PrPC mediated gastric cancer multi-drug-resistance using western blot assay. pAKT was significantly decreased in SGC7901/PRP when transiently transfected with MGr1-Ag/37LRP siRNA compared to siRNA control group and SGC7901 cells (Figure 5C and 5D, p < 0.05). All these results suggest that MGr1-Ag/37LRP might promote PrPC induced gastric cancer multi-drug-resistance by activation of Caspase 3 through the PI3K/AKT pathway.
Figure 5. MGr1-Ag/37LRP promotes PrPC induced gastric cancer multi-drug-resistance by activation of Caspase 3 probably through PI3K/AKT pathway.
(A) and (B) Caspase 3 activation was examined by immunofluorescence staining and the fluorescence intensity was measured using high-content screening scanning. MGr1-Ag/37LRP siRNA significantly increased caspase 3 activation in SGC7901/PrP compared with the control group (*p < 0.05). SGC7901 was employed as a positive control. (C) and (D) Western blot results showed that pAKT were significantly decreased in SGC7901/PRP when transiently transfected with MGr1-Ag/37LRP siRNA (lane 3) compared to the siRNA control group (lane 2, p < 0.05).
DISCUSSION
Gastric cancer is still one of the most common malignancies in China. Adjuvant treatments, which usually comprise chemotherapy, are the main methods for treating advanced gastric cancer patients now. But many patients die of treatment failure, which could be ascribed to a phenomenon known as multi-drug-resistance. As one of the fatal steps of tumor progression, multi-drug-resistance could be a good breakthrough point of research concerning gastric cancer treatment.
PrPC and MGr1-Ag were among the 63 differentially expressed molecules isolated from drug-resistant human gastric adenocarcinoma cell line when compared to its parental cell line in our previous work [10]. Both of them were identified to be involved in promoting the multi-drug-resistance phenotypes of gastric cancer through similar signaling pathways. Overexpression of PrPC in SGC7901 cells demonstrated decreased drug accumulation and increased drug release through upregulating P-glycoprotein, Bcl-2 and downregulating p53 and Bax, [12, 23, 24]. while MGr1-Ag/37LRP might also influence the multi-drug-resistance of gastric cancer cells probably by regulating the expression of P-glycoprotein, Bcl-2 and Bax [20, 25]. In our present study, the expression of these two proteins were found to correlate and co-localized in gastric cancer cell lines and gastric cancer tissues. Co-immunoprecipitation demonstrated that PrPC and MGr1-Ag/37LRP interact with each other and might coexist in the protein complex. Interestingly, other researches also identified 37LRP as a ligand of the PrPC in mammalian and insect cells [14, 15]. Their possible interactive site is located between aa144-179 on PrP, and between aa161-179 on 37LRP [26].
The recycle of PrPC makes it a potential candidate for a ligand uptake, cell adhesion and recognition molecule or a membrane signaling molecule. In our previous work, [27] cDNA microarray analysis aiming to find PrPC-responsive genes in gastric cancer cells showed similar results. Fifty-two adhesion-related genes were shown to be significantly overexpressed (control signal ratio>2.0). It was reported that binding of PrPC to multiple signaling molecules plays a role as a transmitter of information from the extracellular milieu to the cell that subsequently triggers the molecular cascade in multiple cell survival mechanisms. Although we still do not understand all the details, pluralism of PrPC in physiological and pathological processes point to several interconnected pathways [3]. MGr1-Ag/37LRP was verified to promote PrPC mediated multi-drug-resistance in this study. According to our results, siRNA targeting towards MGr1-Ag/37LRP could notably increase the drug sensitivity of PrPC overexpressed SGC7901/PrP cells which showed notable drug resistance in our previous results. Further data demonstrated that MGr1-Ag/37LRP might promote the multi-drug-resistance of SGC7901/PrP mainly by enhancing anti-apoptotic abilities of gastric cancer cells through PI3K/AKT pathway. It was also reported that in cerebral ischemia, PrPC deletion impairs the PI3K/Akt pathway [28]. Llorens F et. al. found that PrPC could modulate epidermal growth factor receptor (EGFR) and enhances cell proliferation and cell cycle reentrance through MAPK-AKT pathway activation [29]. Similarly, in the nervous system, PrPC can also mediate the activation of several signal transduction pathways, including PKA, Fyn, PI3K/Akt, and MAPK/ERK to promote neuronal survival [30, 31]. More interestingly, it was reported that IGF-1-induced enhancement of PrPC expression is due to the activation of the PI3K-Akt signaling pathway.[32] Taken together, we suppose that in PrPC mediated drug resistance, there may be two putative relationships between PrPC and MGr1-Ag/37LRP. On the one hand, MGr1-Ag/37LRP may play a role in signal transduction in extracellular stress trans-membrane to intracellular molecular signal pathways. On the other hand, MGr1-Ag/37LRP may be a synergetic factor of PrPC not only to transfer signals to downstream molecules but also acting as positive feedback to PrPC through activation of PI3K/AKT pathway, thus encouraging the vicious cycle of tumorous drug resistance development. However, more evidence is needed to verify this hypothesis in future work.
As a copper-binding protein expressed predominantly in neurons, PrPC could be induced in ischemic/hypoxic brain tissues and hypoxia could initiate resistance to chemotherapy by altering gene expression in solid tumors. Some transcriptional factors phosphorylated by ERK1/2 could interact with HSE in the promoter of PrPC resulting in upregulation of PrPC in gastric cancer cell line MKN28 during hypoxia [33]. Previous data also showed that FAK-PI3K and p42/44MAPK(ERK1/2) might be the major signaling molecules in MGr1-Ag/37LRP induced HIF-1α expression and activity which contributed to drug resistance and apoptosis resistance in gastric cancer cell lines [34]. These data supports a model of hypoxic stress reaction in gastric cancer cells, in which MGr1-Ag/37LRP might be a focal point of drug resistant signaling pathways.
There were also various studies that showed PrPC as a high affinity laminin ligand for its interaction with synaptic proteins (synapsin Ib and Grb2) and cell adhesion molecules [35, 36]. There is some suggestion that PrPC mediates interactions between the extracellular matrix and the neuron [37]. The 37LRP matures to a 67 kDa protein which facilitates binding of cancer cells to basement membranes [38]. We also identified a role for MGr1-Ag/37LRP in cell adhesion mediated drug resistance (unpublished data). All the data suggests an adhesion molecule related signal pathway as another candidate model of MGr1-Ag/37LRP as a focal point in promoting PrPC related multi-drug-resistance in gastric cancer cells.
Since MGr1 has a novel promoting function on gastric cancer multi-drug-resistance, it could be a potential anti-tumor target which might prevent or delay drug resistance in gastric cancer. Omar et al. predicted that molecular tools such as antibodies directed against LRP/LR have the potential to act as promising alternative therapeutics for the treatment of cancer [39]. We also found that purified MGr1 antibody could significantly increase the 5-FU sensitivity of SGC7901/VCR at a concentration of 20 μg/ml (unpublished data).
Although it needs to be further confirmed in vivo, our findings elucidates the mechanism of how PrPC triggers intracellular signaling cascade resulting in multi-drug-resistance phenotype and provides a novel candidate molecular target against gastric cancer. Combined with the earlier findings gained by our colleagues and other researchers, we propose that MGr1-Ag/37LRP may be a critical factor of several stress associated cellular signal pathways though the precise mechanisms still needs to be determined by further work.
MATERIALS AND METHODS
Cell culture and transfection
SGC7901/VCR cells were generated from the human gastric cancer cell line SGC7901 which was obtained from Academy of Military Medical Science (Beijing, China) by stepwise selection in vitro, using vincristine as inducing reagent. Both SGC7901 and SGC7901/VCR cell lines were preserved in our institute. Cells were cultivated in RPMI1640 medium (Gibco Technologies Inc.) supplemented with 10% fetal bovine serum (Hyclone), 100 U/ml penicillin, and 100 μg/ml streptomycin (Sigma-Aldrich) at 37°C with a humidified atmosphere of 5% CO2 and 95% air, with 1 μg/ml VCR for SGC7901/VCR.
Clinical samples
Formalin-fixed, paraffin-embedded tumor tissue and corresponding normal tissue samples were obtained from 50 gastric cancer patients (aged between 28 to 82 years, 38 males and 12 females, 26 well differentiated and 24 moderately or poorly differentiated) who underwent surgical resection during 2005 and 2006 in Xijing Hospital, Xi'an, China. All patients had not received radiotherapy and chemotherapy before surgery. The protocols were approved by the Ethics Committee of Xijing Hospital. All cases of gastric cancer and adjacent nontumorous tissues were diagnosed clinically and pathologically.
Immunohistochemical staining
MGr1-Ag/37LRP and PrPC immunostaining were performed by an avidin-biotin method as described previously [40]. The expression level of MGr1-Ag/37LRP and PrPC was determined as positive or negative by evaluating the percentage of staining tumor cells: positive for > 25% and negative for < 25%.
Plasmid construction, siRNA and transfection
Stable transfected cell lines SGC7901/PrP and SGC7901/PrPsi were prepared in our previous work [8] by transfecting SGC7901 cells with PCDNA3.1B-PrP plasmids and Psilencer3.1-PrPsiRNA plasmids, respectively. SiRNAs of MGr1-Ag/37LRP were purchased (Shanghai GenePharma Co., Ltd) and selected using western blot (MGr1-Ag/37LRP siRNA sequence: 5′-GUGC AAUUGUUGCCAUUGA-3′-TT; negative control: 5′-UU CUCCGAACGUGUCACGU-3′-TT). Cell transfection was performed with Lipofectamine TM 2000 reagent (Invitrogen) as described in the manufacturer's protocol. Cells transiently transfected with MGr1-Ag/37LRP siRNA or negative control were defined as SGC7901/PrP-37LRPi and SGC7901/PrP-ic, respectively.
Western blot analysis
Comparable protein samples (∼50 μg) were separated on a 12% SDS-PAGE gel and the protein-transferred nitrocellulose (Millipore) was probed with mouse anti-human antibody specific against PrPC (Sigma Company, clone 3F4 bind epitopes comprising 1000), MGr1 (prepared in our lab) [18], AKT/pAKT antibodies (Cell Signaling Technology) or β-actin (Sigma Company). After three washes, the membrane was incubated with horseradish peroxidase-conjugated goat anti-mouse IgG (zhongshan golden bridge biotechnology) as secondary antibodies. The membrane was then washed again and detected using the enhanced chemiluminescence reagent (Bio-Rad Laboratories, Inc.).
Laser scanning confocal fluorescence assay
4% paraformaldehyde-fixed SGC7901 and AGS cells plated on cover slips were permeabilized with 0.2% Triton X-100 and blocked with goat serum for 1 h at room temperature. Cover slips were then incubated with rabbit anti-human PrPC antibody (1:50, Santa Cruz Biotechnology) and MGr1 antibody (1:200) at 4°C overnight. After three washes the cover slips were incubated with FITC-conjugated goat anti-mouse IgG (1:1000, Beyotime Institute of Biotechnology) and Cy3-conjugated goat anti-rabbit IgG (1:1000, Beyotime Institute of Biotechnology) as secondary antibodies for 2 h at room temperature. The nucleus were stained with DAPI. Fluorescent images were collected by the FLUOVIEW laser scanning confocal microscope (Olympus Corp).
Co-immunoprecipitation
Approximately 5 × 107 SGC7901 cells in log phase were lysated with lysis buffer (50 mmol/L Tris-Cl PH = 7.5; 150 mmol/L NaCl; 1% Triton X-100/1% NP-40; 0.5% sodium deoxycholate; 1mM Phenylmethanesulfonyl fluoride (PMSF); 1 μg/ml Aprotinin; 1 μg/ml Leupeptin) and centrifuged. Preclearing of supernatant was performed with 50 μl Protein G Plus/Protein A Agarose (Calbiochem) in dilutus buffer (50 mmol/L Tris-Cl PH = 7.5; 150 mmol/L NaCl; 0.1mg/mL PMSF; 1 μg/ml Aprotinin; 1 μg/ml Leupeptin; 0.1% NP-40). After centrifugation, the supernatant was divided into two parts. One was incubated with monoclonal mouse anti-human PrPC antibody (1:50, Abcam) and the other was incubated with an independent monoclonal mouse anti-human antibody prepared in our laboratory as a negative control for 2 hours. Each of them were added to 50 μl protein A/G-agarose and incubated overnight. The precipitates were collected and carefully washed four times with the abluent buffer I (50 mmol/L Tris-Cl PH=7.5; 150mmol/L NaCl; 0.1% NP-40) and twice with the abluent buffer II (10 mmol/L Tris-Cl PH = 7.5). All the procedures above were conducted at 4°C and with gentle rotation. After centrifugation, pellets were resuspended in 40 μl SDS-PAGE sample buffer (50 mmol/L Tris-Cl pH = 8.8; 2% SDS; 0.1% Bromphenol blue; 10% Glycerol; 1% β-mercaptoethanol) and the immunocomplex supernatant was detected respectively with antibodies specific to PrPC and MGr1 by western blot.
In vitro drug sensitivity assay
Chemotherapeutic drugs frequently used in clinical treatment of gastric cancer (ADR, VCR, CDDP and 5-FU) were chosen to test the drug sensitivity of SGC7901/PrP and SGC7901/VCR derived transfects by the MTT assay. Cells were seeded into 96-well cell culture clusters at a density of 8 × 103 cells/well and cultured for 12 hours before transfection. 12 hours after transient transfection, the medium was replaced with fresh RPMI1640 containing different drug concentrations (10×, 1×, 0.1×, 0.01×, 0×peak value of each drug in blood serum: ADR 0.4 μg/ml, VCR 0.5 μg/ml, CDDP 0.6 μg/ml, 5-FU 2 μg/ml). After 48 hours of growth in the presence of the drugs, 20 μl MTT/well reagent (5mg/ml) was added and the supernatant was discarded after further culturing for 4 hours. Adherent cells were lysed with 150 μl dimethyl sulfoxide. The absorbance of the formazan product was measured with the microplate reader (Model 680, BIO-RAD) at a wavelength of 490 nm. The relative survival rate of cell growth by different concentrations of drugs was calculated according to the following formula: (mean A490 of drug treated wells/mean A490 of untreated wells) ×100%. Finally, dose-dependent curves of drugs were drawn on semilogarithm coordinate paper and IC-50 values were determined. Each study was performed in quadruple and repeated thrice.
Hoechest staining assay
Forty eight hours after transient transfection with MGr1-Ag/37LRP siRNA or control siRNA, respectively, SGC7901/PrP and SGC7901 cells were induced by VCR in a final concentration of 1 μg/ml for 18 hours except for blank control group. Hoechest staining was performed with a Hoechest staining assay kit (Beyotime Institute of Biotechnology) as described in the manufacturer's protocol. Each experiment was independently repeated thrice.
Analysis of apoptosis by flow cytometer
SGC7901/PrP cells were transiently transfected with MGr1-Ag/37LRP siRNA or control siRNA, respectively, and were further cultured for 48 hours. Then SGC7901/PrP and SGC7901 cells were induced by VCR in a final concentration of 1 μg/ml for 18 hours except for blank control group. The cells were suspended in 200 μl Binding Buffer and 10 μl Annexin V-FITC and 5 μl PI were added. Apoptotic cell numbers were counted with a flow cytometer. A relative apoptotic rate was calculated according to the following formula: the amount of apoptotic cells (FITC+ and PI−) per 6000 cells/6000 cells counted randomly×100%. Each experiment was independently repeated thrice.
Caspase 3 activation detecting and quantitating assay
Activated caspase 3 detecting and quantitating assay was performed by a high-content screening method that allows direct in-cell measurements using a fixed end-point assay based on immunofluorescence detection in cells grown on standard high-density microplates. SGC7901/PrP or SGC7901 cells were seeded into 96-well cell culture clusters at a density of 8×103 cells/well and incubated for 12 hours at 37°C in 5% CO2. SGC7901/PrP cells were then transiently transfected with MGr1-Ag/37LRP siRNA or control siRNA, respectively, and further cultured for 48 hours. All cells were induced by VCR in a final concentration of 1 μg/ml for 18 hours. Activated caspase 3 (cleaved) detecting and quantitating assay was performed with Cellomics® Caspase 3 Activation Kit (Thermo Scientific) using Thermo Scientific ArrayScan® VTI HCS Reader as described in the manufacturer's protocol. Each study was performed in quadruple and independently repeated thrice.
Statistical analysis
Numerical data are presented as the means ± SD/SEM. All statistical analyses were performed using GraphPad Prism 5. The differences between groups were assessed by the Student's t-test when comparing only two groups or one-way analysis of variance when more than two groups were compared. Chi-square test was used in correlation analysis of immunohistochemical results. P < 0.05 was considered as statistically significant and all reported p values were two-sided.
Acknowledgments
We thank Taidong Qiao, Zheng Chen, Yifeng Peng, Jintao Hu and Yunxin Cao for their excellent technical assistance. We also thank Ukpong B. Eyo, Yamei Wu and Julin Ren for their warm hearted help.
Footnotes
CONFLICTS OF INTEREST
None.
GRANT SUPPORT
This work was supported by the National Natural Science Foundation of China (grant numbers 81322037, 81572302, 81421003)
REFERENCES
- 1.McKinley MP, Bolton DC, Prusiner SB. A protease-resistant protein is a structural component of the scrapie prion. Cell. 1983;35:57–62. doi: 10.1016/0092-8674(83)90207-6. [DOI] [PubMed] [Google Scholar]
- 2.Prusiner SB, DeArmond SJ. Prion diseases of the central nervous system. Monogr Pathol. 1990:86–122. [PubMed] [Google Scholar]
- 3.Lasmezas CI. Putative functions of PrP(C) Br Med Bull. 2003;66:61–70. doi: 10.1093/bmb/66.1.61. [DOI] [PubMed] [Google Scholar]
- 4.Kim BH, Lee HG, Choi JK, Kim JI, Choi EK, Carp RI, Kim YS. The cellular prion protein (PrPC) prevents apoptotic neuronal cell death and mitochondrial dysfunction induced by serum deprivation. Brain Res Mol Brain Res. 2004;124:40–50. doi: 10.1016/j.molbrainres.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 5.Cui HL, Guo B, Scicluna B, Coleman BM, Lawson VA, Ellett L, Meikle PJ, Bukrinsky M, Mukhamedova N, Sviridov D, Hill AF. Prion infection impairs cholesterol metabolism in neuronal cells. J Biol Chem. 2014;289:789–802. doi: 10.1074/jbc.M113.535807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Watt NT, Griffiths HH, Hooper NM. Neuronal zinc regulation and the prion protein. Prion. 2013;7:203–8. doi: 10.4161/pri.24503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Singh A, Haldar S, Horback K, Tom C, Zhou L, Meyerson H, Singh N. Prion protein regulates iron transport by functioning as a ferrireductase. J Alzheimers Dis. 2013;35:541–52. doi: 10.3233/jad-130218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pan Y, Zhao L, Liang J, Liu J, Shi Y, Liu N, Zhang G, Jin H, Gao J, Xie H, Wang J, Liu Z, Fan D. Cellular prion protein promotes invasion and metastasis of gastric cancer. Faseb j. 2006;20:1886–8. doi: 10.1096/fj.06-6138fje. [DOI] [PubMed] [Google Scholar]
- 9.Liang J, Pan Y, Zhang D, Guo C, Shi Y, Wang J, Chen Y, Wang X, Liu J, Guo X, Chen Z, Qiao T, Fan D. Cellular prion protein promotes proliferation and G1/S transition of human gastric cancer cells SGC7901 and AGS. Faseb j. 2007;21:2247–56. doi: 10.1096/fj.06-7799com. [DOI] [PubMed] [Google Scholar]
- 10.Zhao Y, You H, Liu F, An H, Shi Y, Yu Q, Fan D. Differentially expressed gene profiles between multidrug resistant gastric adenocarcinoma cells and their parental cells. Cancer Lett. 2002;185:211–8. doi: 10.1016/s0304-3835(02)00264-1. [DOI] [PubMed] [Google Scholar]
- 11.Zheng G, Liu X, Han F. [Establishment and characterization of doxorubicin-resistant BGC-823/DOX of human gastric cancer cell line] Zhonghua Wai Ke Za Zhi. 1997;35:262–4. [PubMed] [Google Scholar]
- 12.Du J, Pan Y, Shi Y, Guo C, Jin X, Sun L, Liu N, Qiao T, Fan D. Overexpression and significance of prion protein in gastric cancer and multidrug-resistant gastric carcinoma cell line SGC7901/ADR. Int J Cancer. 2005;113:213–20. doi: 10.1002/ijc.20570. [DOI] [PubMed] [Google Scholar]
- 13.Marc D, Mercey R, Lantier F. Scavenger, transducer, RNA chaperone? What ligands of the prion protein teach us about its function. Cell Mol Life Sci. 2007;64:815–29. doi: 10.1007/s00018-007-6370-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rieger R, Edenhofer F, Lasmezas CI, Weiss S. The human 37-kDa laminin receptor precursor interacts with the prion protein in eukaryotic cells. Nat Med. 1997;3:1383–8. doi: 10.1038/nm1297-1383. [DOI] [PubMed] [Google Scholar]
- 15.Gauczynski S, Peyrin JM, Haik S, Leucht C, Hundt C, Rieger R, Krasemann S, Deslys JP, Dormont D, Lasmezas CI, Weiss S. The 37-kDa/67-kDa laminin receptor acts as the cell-surface receptor for the cellular prion protein. Embo j. 2001;20:5863–75. doi: 10.1093/emboj/20.21.5863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morel E, Andrieu T, Casagrande F, Gauczynski S, Weiss S, Grassi J, Rousset M, Dormont D, Chambaz J. Bovine prion is endocytosed by human enterocytes via the 37 kDa/67 kDa laminin receptor. Am J Pathol. 2005;167:1033–42. doi: 10.1016/s0002-9440(10)61192-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kolodziejczak D, Da Costa Dias B, Zuber C, Jovanovic K, Omar A, Beck J, Vana K, Mbazima V, Richt J, Brenig B, Weiss SF. Prion interaction with the 37-kDa/67-kDa laminin receptor on enterocytes as a cellular model for intestinal uptake of prions. J Mol Biol. 2010;402:293–300. doi: 10.1016/j.jmb.2010.06.055. [DOI] [PubMed] [Google Scholar]
- 18.Shi Y, Zhai H, Wang X, Wu H, Ning X, Han Y, Zhang D, Xiao B, Wu K, Fan D. Multidrug-resistance-associated protein MGr1-Ag is identical to the human 37-kDa laminin receptor precursor. Cell Mol Life Sci. 2002;59:1577–83. doi: 10.1007/s00018-002-8531-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu L, Sun L, Zhang H, Li Z, Ning X, Shi Y, Guo C, Han S, Wu K, Fan D. Hypoxia-mediated up-regulation of MGr1-Ag/37LRP in gastric cancers occurs via hypoxia-inducible-factor 1-dependent mechanism and contributes to drug resistance. Int J Cancer. 2009;124:1707–15. doi: 10.1002/ijc.24135. [DOI] [PubMed] [Google Scholar]
- 20.Sun L, Shi Y, Guo C, Yao L, Lin T, Du J, Han Q, Han Y, Fan D. Regulation of multidrug resistance by MGr1-antigen in gastric cancer cells. Tumour Biol. 2006;27:27–35. doi: 10.1159/000090153. [DOI] [PubMed] [Google Scholar]
- 21.Castronovo V, Taraboletti G, Sobel ME. Functional domains of the 67-kDa laminin receptor precursor. J Biol Chem. 1991;266:20440–6. [PubMed] [Google Scholar]
- 22.Liang J, Ge F, Guo C, Luo G, Wang X, Han G, Zhang D, Wang J, Li K, Pan Y, Yao L, Yin Z, Guo X, et al. Inhibition of PI3K/Akt partially leads to the inhibition of PrP(C)-induced drug resistance in gastric cancer cells. Febs j. 2009;276:685–94. doi: 10.1111/j.1742-4658.2008.06816.x. [DOI] [PubMed] [Google Scholar]
- 23.Liang J, Pan YL, Ning XX, Sun LJ, Lan M, Hong L, Du JP, Liu N, Liu CJ, Qiao TD, Fan DM. Overexpression of PrPC and its antiapoptosis function in gastric cancer. Tumour Biol. 2006;27:84–91. doi: 10.1159/000092488. [DOI] [PubMed] [Google Scholar]
- 24.Du JP, Jin XH, Shi YQ, Zhao YQ, Liu CJ, Cao YX, Qiao TD, Chen BJ, Fan DM. [The overexpression of prion protein in drug resistant gastric cancer cell line SGC7901/ADR and its significance] Zhonghua Yi Xue Za Zhi. 2003;83:328–32. [PubMed] [Google Scholar]
- 25.Shi Y, Han Y, Wang X, Zhao Y, Ning X, Xiao B, Fan D. MGr1-Ag is associated with multidrug-resistant phenotype of gastric cancer cells. Gastric Cancer. 2002;5:154–9. doi: 10.1007/s101200200027. [DOI] [PubMed] [Google Scholar]
- 26.Hundt C, Peyrin JM, Haik S, Gauczynski S, Leucht C, Rieger R, Riley ML, Deslys JP, Dormont D, Lasmezas CI, Weiss S. Identification of interaction domains of the prion protein with its 37-kDa/67-kDa laminin receptor. Embo j. 2001;20:5876–86. doi: 10.1093/emboj/20.21.5876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liang J, Luo G, Ning X, Shi Y, Zhai H, Sun S, Jin H, Liu Z, Zhang F, Lu Y, Zhao Y, Chen X, Zhang H, et al. Differential expression of calcium-related genes in gastric cancer cells transfected with cellular prion protein. Biochem Cell Biol. 2007;85:375–83. doi: 10.1139/o07-052. [DOI] [PubMed] [Google Scholar]
- 28.Weise J, Sandau R, Schwarting S, Crome O, Wrede A, Schulz-Schaeffer W, Zerr I, Bahr M. Deletion of cellular prion protein results in reduced Akt activation, enhanced postischemic caspase-3 activation, and exacerbation of ischemic brain injury. Stroke. 2006;37:1296–300. doi: 10.1161/01.STR.0000217262.03192.d4. [DOI] [PubMed] [Google Scholar]
- 29.Llorens F, Carulla P, Villa A, Torres JM, Fortes P, Ferrer I, del Rio JA. PrP(C) regulates epidermal growth factor receptor function and cell shape dynamics in Neuro2a cells. J Neurochem. 2013;127:124–38. doi: 10.1111/jnc.12283. [DOI] [PubMed] [Google Scholar]
- 30.Mouillet-Richard S, Ermonval M, Chebassier C, Laplanche JL, Lehmann S, Launay JM, Kellermann O. Signal transduction through prion protein. Science. 2000;289:1925–8. doi: 10.1126/science.289.5486.1925. [DOI] [PubMed] [Google Scholar]
- 31.Chen S, Mange A, Dong L, Lehmann S, Schachner M. Prion protein as trans-interacting partner for neurons is involved in neurite outgrowth and neuronal survival. Mol Cell Neurosci. 2003;22:227–33. doi: 10.1016/s1044-7431(02)00014-3. [DOI] [PubMed] [Google Scholar]
- 32.Liu T, Yi W, Feng B, Zhou Z, Xiao G. IGF-1-induced enhancement of PRNP expression depends on the negative regulation of transcription factor FOXO3a. PLoS One. 2013;8:e71896. doi: 10.1371/journal.pone.0071896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liang J, Bai F, Luo G, Wang J, Liu J, Ge F, Pan Y, Yao L, Du R, Li X, Fan R, Zhang H, Guo X, et al. Hypoxia induced overexpression of PrP(C) in gastric cancer cell lines. Cancer Biol Ther. 2007;6:769–74. doi: 10.4161/cbt.6.5.4001. [DOI] [PubMed] [Google Scholar]
- 34.Liu L, Zhang H, Sun L, Gao Y, Jin H, Liang S, Wang Y, Dong M, Shi Y, Li Z, Fan D. ERK/MAPK activation involves hypoxia-induced MGr1-Ag/37LRP expression and contributes to apoptosis resistance in gastric cancer. Int J Cancer. 2010;127:820–9. doi: 10.1002/ijc.25098. [DOI] [PubMed] [Google Scholar]
- 35.Schmitt-Ulms G, Legname G, Baldwin MA, Ball HL, Bradon N, Bosque PJ, Crossin KL, Edelman GM, DeArmond SJ, Cohen FE, Prusiner SB. Binding of neural cell adhesion molecules (N-CAMs) to the cellular prion protein. J Mol Biol. 2001;314:1209–25. doi: 10.1006/jmbi.2000.5183. [DOI] [PubMed] [Google Scholar]
- 36.Spielhaupter C, Schatzl HM. PrPC directly interacts with proteins involved in signaling pathways. J Biol Chem. 2001;276:44604–12. doi: 10.1074/jbc.M103289200. [DOI] [PubMed] [Google Scholar]
- 37.McBride SM. Prion protein: a pattern recognition receptor for viral components and uric acid responsible for the induction of innate and adaptive immunity. Med Hypotheses. 2005;65:570–7. doi: 10.1016/j.mehy.2005.02.038. [DOI] [PubMed] [Google Scholar]
- 38.Jaseja M, Mergen L, Gillette K, Forbes K, Sehgal I, Copie V. Structure-function studies of the functional and binding epitope of the human 37 kDa laminin receptor precursor protein. J Pept Res. 2005;66:9–18. doi: 10.1111/j.1399-3011.2005.00267.x. [DOI] [PubMed] [Google Scholar]
- 39.Omar A, Jovanovic K, Da Costa Dias B, Gonsalves D, Moodley K, Caveney R, Mbazima V, Weiss SF. Patented biological approaches for the therapeutic modulation of the 37 kDa/67 kDa laminin receptor. Expert Opin Ther Pat. 2011;21:35–53. doi: 10.1517/13543776.2011.539203. [DOI] [PubMed] [Google Scholar]
- 40.Lu Y, Wang X, Liu Z, Jin B, Chu D, Zhai H, Zhang F, Li K, Ren G, Miranda-Vizuete A, Guo X, Fan D. Identification and distribution of thioredoxin-like 2 as the antigen for the monoclonal antibody MC3 specific to colorectal cancer. Proteomics. 2008;8:2220–9. doi: 10.1002/pmic.200700770. [DOI] [PubMed] [Google Scholar]