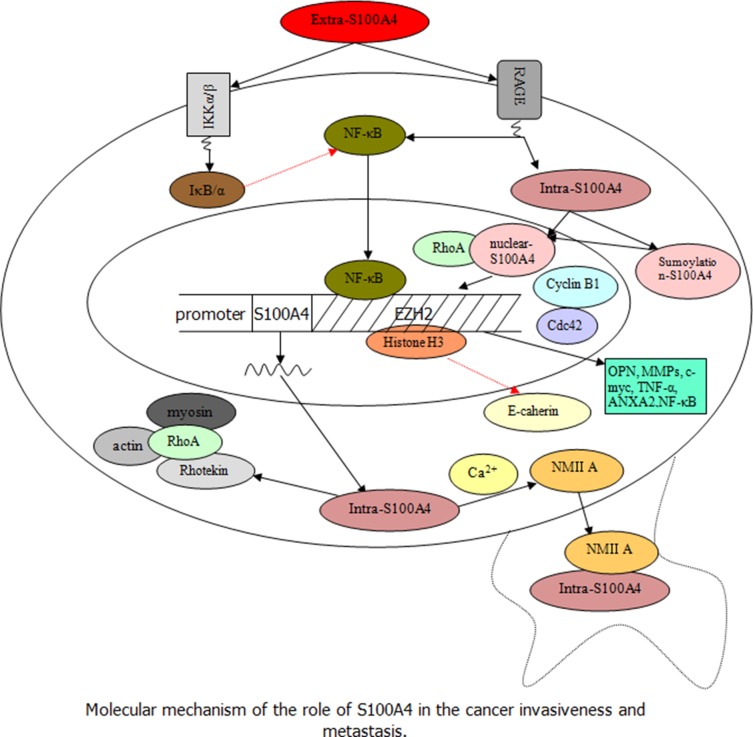
Figure 2. Speculation about the role of S100A4 in the regulation and promotion of tumor progression and metastasis (Black arrow indicates the promoting effect and red dashed arrow indicates the inhibitory effect).
Extra-S100A4 derived from tumor and stromal cells activates the transcription factor NF-κB, not only by regulating the RAGE but also by inducing phosphorylation of IKK α/β leading to increased phosphorylation of IκB α (inhibitor protein of NF-κB). Besides, the nuclear translocation of intra-S100A4 through RAGE-dependent regulation and sumoylation-mediated signaling can trigger the downstream signal cascades of S100A4 to secrete several molecules such as OPN, MMPs, c-myc, TNF-α, and ANXA2, associated with tumor cell invasion, which cooperates with RhoA, cdc 42, cyclin B1, and NF-κB. In addition, the intra-S100A4 activated by Ca2+ could regulate the stability of lamellipodia and enhance cell migration by interacting with NMIIA; intra-S100A4 is also able to combine with the Rhotekin–RhoA complex to promote membrane ruffling and invasion by coupling of myosin and actin, which are associated with an increase in formation of tumor cell spread and metastasis.