Abstract
Objectives
Maintaining regular physical activity (PA) delays the onset of functional disability and preserves mobility later in life. This study examined prospective associations between changes in PA and changes in physical performance measures (PPMs) over 6-years in older women.
Design
Prospective Cohort Study
Setting
Forty clinical centers in the United States
Participants
Women aged 65 years and older (mean, 69.8 y) enrolled in the Women’s Health Initiative Clinical Trials with gait speed, timed chair stand, grip strength, and self-reported recreational PA data assessed at baseline (1993–1998) and at follow-up years 1, 3 and 6 (N=5092).
Measurements
Mixed effects linear regression models described the association between time-varying PA and change in each PPMs. Potential interactions between time-varying PA and age (<70 y; ≥70 y) were also tested.
Results
Significant, dose-response associations between PA and improvements in all PPMs were observed over the 6 y follow-up after adjusting for important covariates. Compared to sedentary women (<100 MET-min/wk), high PA groups (≥1200 MET-min/wk) had better grip strength (0.48 kg higher; P<0.01), more chair stands (0.35 more; P<0.001) and faster gait speeds (0.06 m/s faster; P<0.001). Higher PA levels were associated with a greater increase in chair stands in women aged ≥70 y (P<0.001) than the younger group (Pinteraction for age=0.014).
Conclusions
In post-menopausal women, maintaining high PA levels over time is associated with benefits in lower extremity function, compared to remaining sedentary. These data support the view that regular PA plays an important role in maintaining functional status during aging in older women.
Keywords: mobility disability, physical performance, physical activity, postmenopausal women, epidemiology
INTRODUCTION
The number of Americans aged ≥ 65 y is expected to increase to 88.5 million (56% women) by 2030, which may increase the public health burden of chronic illnesses, injuries, functional limitations and disabilities.1 Age-related mobility impairments may also compromise quality of life, thus, maintaining physical function may be of particular importance in older people. There is, therefore, considerable interest in the links between performance-based measures of physical function and frailty, loss of physiological reserves, likelihood of illness, and premature mortality among older adults.2 Physical activity (PA) has been identified as a behavioral, intra-individual determinant of disablement in older adults and is considered a primary determinant of preventing functional limitations.3,4 PA has also been purported to enhance health and improve functional outcomes, including skeletal muscle strength, endurance, and aerobic capacity,3,5 and thus, may be instrumental in minimizing or preventing mobility disability in older adults.3,4,6
Despite reported benefits of PA on physical function and quality of life in older adults,7,8 few studies have examined late-life PA longitudinally, beyond its relationship to chronic disease and overall morbidity.2,8,9 To date, most studies have been cross-sectional,10,11 based on baseline PA levels, or of short duration. How changes in PA levels over time relate to changes in physical functioning over an extended follow-up is unclear in older women. The Women’s Health Initiative (WHI) cohort provided the opportunity to evaluate the prospective relationships between 6-year changes in PA and physical performance measures (PPMs) of balance, mobility (gait), and muscle strength, in post-menopausal women.
METHODS
Study Population
The WHI study design, eligibility criteria, data collection, and outcomes ascertainment and adjudication have been published.12,13 In brief, 161,808 postmenopausal women (aged 50–79 y, mean age 63 y) were recruited at 40 U.S, clinical centers between 1993 and 1998 to enroll in one or more of three clinical trials (CT: menopausal hormone therapy, diet modification, and calcium/vitamin D supplementation) or an observational study (OS). The study population for the present analysis arose from a random sample of 5962 (25%) older CT women (age ≥ 65 y) who completed performance-based physical function tests at baseline, 5520 of whom had data for self-reported PA and three specific PPMs (i.e., timed walk speed, single/repeated chair stand, and hand grip strength). Of these, 348 were excluded for not having at least 1 follow-up measure of each PPM, and an additional 80 were excluded for lacking an accompanying PA report at year 3 or 6, resulting in a final analytic sample size of 5092 women, all of whom provided informed consent. Protocols were approved by appropriate institutional review boards.
Measures
Assessment of recreational PA in the WHI has been described previously.14,15 In brief, PA was assessed by questions on the frequency and duration of walking and recreational activities, and summarized as PA energy expenditure using standardized metabolic equivalent (MET) intensity values, computed as the product of days per week, minutes per day, and MET score for each activity.16 MET intensity values were assigned for strenuous, moderate-, and light-intensity activities as 7, 4, and 3 METs, respectively, based on the most recent Compendium of Physical Activities (2011).17 Four speed categories were used: less than 2 mph (casual strolling or walking, 2.0 MET), 2–3 mph (average or normal walking, 3.0 MET), 3–4 mph (fairly fast walking, 4.0 MET), or more than 4 mph (very fast walking, 4.5 MET). Information on walking and recreational PA was used to generate a summary total PA variable in MET-hr/wk (which multiplied by 60 can then be converted to MET-min/wk). Women were classified into four PA groups at baseline, year 3, and year 6, separately: sedentary, which included women reporting no recreational PA or very low PA levels (<100 MET-min/wk), low PA (100–<500 MET-min/wk), moderate PA (500–<1200 MET-min/wk), and high PA (≥1200 MET-min/wk). As stated in a prior WHI report,14 achieving a minimum of 500 MET-min/wk meets current national guidelines to engage in at least 150 min/wk of moderate-intensity PA (i.e. the minimum health-related dose of activity recommended by the 2008 US Physical Activity Guidelines).18
Standard PPMs were assessed at baseline and at years 1, 3, and 6 by trained, certified study staff using standard protocols. The reliability, sensitivity to change, and predictive validity of timed gait speed (m/s), chair stand (number) and grip strength (kg) have been published.19,20 Gait speed was assessed by measuring the time in seconds that it took to complete a 6-meter walk, performed at usual pace, using ambulatory aids as needed. The test was repeated, and the faster of the two measured times was included in this analysis. Chair stands were conducted if the participant was able to stand at least once, without using hands or arms, from a straight-backed, non-padded, flat-seated, armless chair. The number of chair rises able to be performed in 15 seconds was measured to assess lower extremity muscle strength and balance. Two 15-second trials of repeated chair stands were performed with arms folded across the chest, with a 1–2 minutes rest between trials, and the score with the greater number of chair stands was included in this analysis. Grip strength, tested on the dominant hand, measured voluntary muscle strength using a hydraulic hand grip dynamometer (Jamar hand dynamometer; Lafayette Instruments, Lafayette, IN). The participant was instructed to squeeze the handle of the dynamometer as hard as she could during two trials, with staff coaching, and the higher score was used in this analysis. Baseline demographic characteristics, medical history and self-reported general health, falls in the past 12 months, and depression were collected via standard self-report assessments at baseline. Self-reported physical functioning was assessed by SF-36 subscale of the Rand-36 (SF-36).21 Anthropometrics were assessed at each clinic visit. BMI was calculated as weight (kg) / height (m)2. Data collection methods are described elsewhere.13
Statistical Analysis
Baseline demographic and health characteristics were compared across categories of self-reported PA using chi (χ2) tests and ANOVA for categorical and continuous variables, respectively. The primary outcomes were the changes from baseline in each of three PPMs: gait speed; chair stands; and grip strength. The primary independent variable of interest was self-reported PA at baseline and at years 3 and 6. PA was incorporated in a time-varying fashion using methods described previously.14 Briefly, for the difference in outcome at the year 1 clinical visit, baseline PA was used. Similarly, at the year 3 and year 6 visits, PA reported on year 3 and year 6 questionnaires, respectively, were used. Mixed effects linear regression analysis was applied to describe the association between time-varying PA and change in each PPM over the 6-year study period. This modeling approach accounts for the correlation of responses within a subject over time by including a subject-specific random intercept in the model. Adjustment for covariates were considered for any measures or characteristics, or reported clinical or medical factor present at baseline, that may potentially confound the relationship between PA and PPM, based on previous literature.14,22 Thus, three levels of covariate adjustment were applied to all models: Model 1 was adjusted for age at baseline, age at menopause, and baseline PPM (i.e., timed gait speed, chair stands, or grip strength); Model 2 was further adjusted for race/ethnicity, education, current employment, alcohol use, smoking, BMI, depression, and clinical trial arm(s); and Model 3 was further adjusted for history of hypertension, diabetes, cardiovascular disease, and arthritis.
Likelihood ratio tests were used to assess potential interactions between time-varying PA and race/ethnicity or age (<70 y; ≥70 y). Sensitivity analyses were employed excluding women who reported having a disease that may affect physical functioning, namely walking ability or speed (about 15% of sample excluded): emphysema, angina, myocardial infarction (MI), peripheral arterial disease (PAD), transient ischemic attack (TIA), Parkinson’s disease, multiple sclerosis (MS), and amyotrophic lateral sclerosis (ALS). Statistical analyses were conducted using Stata 14.1 (StataCorp, College Station, TX); all reported P-values are two-sided.
RESULTS
At baseline, women were on average (mean ± SD) 69.8 ± 3.7 y (range, 65–81 y), 23.0% women were categorized as sedentary, 30.4% as having low PA levels, 27.5% as moderate PA levels; and 19.1% as high PA levels. Of note, among women in the sedentary group, 69% (n=807) reported engaging in 0 MET-min/wk of PA. Women in the sedentary or low PA groups reported earlier onset of menopause, larger waist circumference, and higher BMI, than higher-PA groups (see Supplementary Table S1; all P < 0.01). Sedentary and low PA women self-reported lower physical functioning, and a greater proportion of these women reported having fair or poor health and history of hypertension, diabetes, cardiovascular disease, and arthritis compared to moderate and high activity PA groups (all P < 0.01).
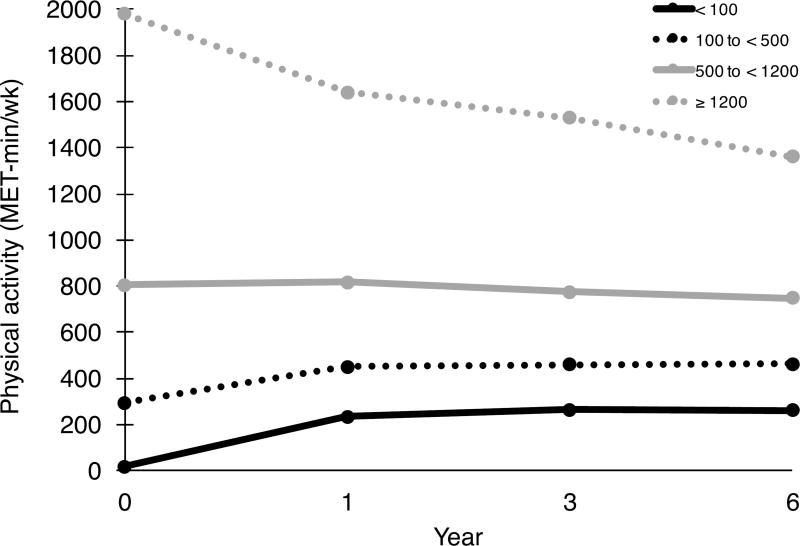
Overall, participants reported significant increases in PA, averaging 38.6 (± 684) MET-min/wk, from baseline to the 1-year follow up (P=0.001); whereas, at year 6, self-reported PA levels decreased on average by 28.6 (±776) MET min/wk from baseline (P=0.012; see Supplementary Table S2). Trajectories in PA differed across assessments depending on initial reported PA level (Figure 1). Women in moderate and high baseline PA groups decreased activity significantly over time (P<0.001); whereas, women in sedentary and low baseline PA groups reported higher activity levels at years 1, 3 and 6 (all P <0.001), yet reported substantially lower levels than the women in moderate and high baseline PA groups at all time points.
Figure 1.
Self-reported physical activity levels across time, stratified by baseline physical activity category (MET-min/wk): Sedentary, < 100; Low, 100 to < 500; Moderate, 500 to < 1200; or High ≥ 1200. Change from baseline to year 6: < 100, 242 ± 455; 100 to < 500, 170 ± 545; 500 to < 1200, –56.2 ± 692; ≥ 1200, –613 ± 1107; all P<0.05
Positive changes in each PPM assessed were associated with time-varying PA over the 6-year follow-up (Table 1). Although the greatest gains in PPMs were seen in the high PA women, even those in the low-PA group (100 to < 500 MET-min/wk) had higher PPM measures than sedentary women over the 6 year follow-up. Specifically, the adjusted OR (95% CI) for low-PA and high-PA groups showed 0.29 (0.02–0.55) and 0.48 (0.15–0.80) kg better grip strength, respectively, than sedentary women. Likewise, compared to sedentary women, low-, moderate-, and high-PA women had 0.14 (0.06–0.23), 0.28 (0.19, 0.37), and 0.35 (0.25–0.46) more chair stands, respectively, and 0.02 (0.01–0.03), 0.04 (0.02, 0.05), and 0.06 (0.04–0.07) m/s faster gait speed.
Table 1.
Association between time-varying physical activity and change in physical function using mixed effects linear regression
| Physical function measure |
Physical activity (MET- min/wk)a |
Model 1 β (95% CI) |
Model 2 β (95% CI) |
Model 3 β (95% CI) |
|---|---|---|---|---|
| Grip strength (kg) | ||||
| < 100 | Ref. | Ref. | Ref. | |
| 100 to < 500 | 0.31 (0.05, 0.57) | 0.28 (0.01, 0.54) | 0.29 (0.02, 0.55) | |
| 500 to < 1200 | 0.32 (0.05, 0.59) | 0.25 (–0.03, 0.53) | 0.25 (–0.03, 0.54) | |
| ≥ 1200 | 0.53 (0.22, 0.84) | 0.49 (0.17, 0.81) | 0.48 (0.15, 0.80) | |
| Chair stands (n) | ||||
| < 100 | Ref. | Ref. | Ref. | |
| 100 to < 500 | 0.20 (0.12, 0.28) | 0.14 (0.06, 0.22) | 0.14 (0.06, 0.23) | |
| 500 to < 1200 | 0.37 (0.28, 0.45) | 0.29 (0.21, 0.38) | 0.28 (0.19, 0.37) | |
| ≥ 1200 | 0.45 (0.35, 0.55) | 0.36 (0.26, 0.47) | 0.35 (0.25, 0.46) | |
| Gait speed (m/s) | ||||
| < 100 | Ref. | Ref. | Ref. | |
| 100 to < 500 | 0.02 (0.01, 0.04) | 0.02 (0.01, 0.03) | 0.02 (0.01, 0.03) | |
| 500 to < 1200 | 0.05 (0.04, 0.06) | 0.04 (0.03, 0.05) | 0.04 (0.02, 0.05) | |
| ≥ 1200 | 0.07 (0.06, 0.08) | 0.06 (0.04, 0.07) | 0.06 (0.04, 0.07) |
CI, confidence interval
Sedentary (<100); Low (100 to < 500); Moderate (500 to < 1200); High (≥1200)
Model 1 adjusted for age at menopause, and baseline physical function measure
Model 2 further adjusted for race/ethnicity, education, current employment, alcohol use, smoking, BMI, depression, and clinical trial arm(s)
Model 3 further adjusted for history of hypertension, diabetes, cardiovascular disease, and arthritis
There was a significant interaction between time-varying PA and age (each as continuous variables) on the number of chair stands (Pinteraction for age = 0.014), but not grip strength or gait speed. The association between time-varying PA and chair stands was subsequently stratified by mean age of 70 y (Table 2). The positive association between time-varying PA and chair stands appeared to be larger in women ≥70 y than women <70 y, with older, high-PA women demonstrating 0.48 (0.32–0.65) more chair stands compared to sedentary women (P<0.001). In contrast, among women aged <70 y, high-PA women showed a more modest 0.24 (0.11–0.38) increase in chair stands than sedentary women (P<0.001).
Table 2.
Association between time-varying physical activity and change in the number of chair stands, by agea
| Physical activity (MET-min/wk)b |
Age <70 y β (95% CI) |
Age ≥70 y β (95% CI) |
|---|---|---|
| < 100 | Ref. | Ref. |
| 100 to < 500 | 0.10 (–0.01, 0.20) | 0.19 (0.07, 0.32) |
| 500 to < 1200 | 0.25 (0.13, 0.37) | 0.31 (0.17, 0.44) |
| ≥ 1200 | 0.24 (0.11, 0.38) | 0.48 (0.32, 0.65) |
CI, confidence interval
All estimates adjusted for Model 3 covariates: age at menopause, baseline physical function measure, race/ethnicity, education, current employment, alcohol use, smoking, BMI, depression, clinical trial arm, and history of hypertension, diabetes, cardiovascular disease, arthritis
Sedentary (<100); Low (100 to < 500); Moderate (500 to < 1200); High (≥1200)
Sensitivity analyses that excluded women with personal history of emphysema, angina, PAD, MS, ALS, MI, TIA, did not substantially change the results (data not shown).”
DISCUSSION
In this analysis of women initially aged 65–79, positive associations between time-varying self-reported recreational PA and three key physical performance measures, i.e. grip strength, chair stands, and gait speed over 6 years were demonstrated, independent of several potential confounding variables. In particular, an increase in PA was associated an increased number of chair stands in 15 seconds across the age continuum, particularly in women aged 70 and over. Unfortunately, physical inactivity generally increases with age,4,23,24 especially in older women.7,8 In the present study, maintaining PA, compared to a drop in PA at all levels, was also associated with improved physical functioning over time.
In one of the largest follow-up studies to date, Brach et al. showed that women who were reportedly “always active,” assessed during a 14-year period, had the highest functional status at the 14-year follow-up visit, compared to “never inactive” or “inconsistently active” women.8 The current study expands and confirms previous epidemiologic observations evaluating PA over time by measuring PA at multiple time points with accompanying PPMs at years 3 and 6. PA levels were categorized in terms of frequency, duration and intensity represented as MET-min/wk (i.e., energy expenditure), making it relevant to national PA guidelines.18 These results could help guide future intervention studies that aim to increase physical function by encouraging more frequent PA at MET intensity levels that are appropriate to and achievable by older women.
Whereas a 1-year change (usually a decline) of 0.1m/s in 6–meter gait speed is considered to be clinically meaningful,25 the modest increases in gait speed (0.02–0.06 m/s) with higher PA have not yet been similarly validated. Slower sit-to-stand times have previously been related to greater deficits in instrumental activities of daily living and to balance disorders in older adults.26 Along these lines, our robust association between increased PA and improved chair stand performance over time among women ≥70 y than women <70 y is unique such as it suggests that late-life PA maintained during aging may be associated with better lower extremity proprioception, strength, and balance outcomes26. Other large cohort studies have shown that higher levels of PA performed midlife were associated better lower extremity function and mobility in older adults (70 y and older)6,8,11Although retrospective PA data was not included in these analyses, it possible that women ≥70 y initiated PA earlier in life and continued PA throughout older adulthood, translating into greater lower-extremity functional status. Likewise, improved chair stand performance may have been derived from daily chores, housework or family care that are not traditionally considered (or reported) as PA. Future research should focus on establishing whether chair stands and grip strength are also similarly predictive of daily life impairments. A recent study involving 17 countries showed that 1-SD decrease in grip strength was a significant prognostic indicator of cardiovascular mortality, even stronger than systolic blood pressure, in older adults over a 4-y follow-up.27 Health outcomes of this type may be related to increases grip strength, chair stands and walking speed increases like those observed in the low and high PA groups, compared to sedentary women and will be examined in the WHI. Some support comes from a cross-sectional analysis in 60–64 year olds showing that lower chair rise performance or lower grip strength was significantly associated with several chronic diseases.28 Of course, low PA levels may be due to a greater preponderance of physical limitations and/or health problems which increase sedentary behavior. In the present study, women in the sedentary and low-PA groups had a higher prevalence of hypertension, diabetes, cardiovascular disease, and arthritis at baseline compared to moderate- and high-PA women and although we adjusted for these conditions, we cannot address the causative nature of the relationships. However, sensitivity analyses were performed, excluding of women (approximately 15% of the study sample) with reported history emphysema, angina, PAD, MS, ALS, MI, TIA and results did not substantially change (data not shown). For this reason, we did not explore adjustment for these clinical events during follow-up. Nonetheless, our data are consistent with the hypothesis that maintaining even low PA during aging could attenuate age-associated, modifiable chronic diseases. For example, regular walking of 8 blocks per week was shown to be protective against further mobility loss in functionally limited women (≥ 65 y) and was associated with improved usual gait speed over one year.9 Thus, while engagement in regular moderate-to-vigorous PA may be associated with more optimal changes in functional status, engaging in regular PA at lower levels also appears to confer modest benefits in functional capacity in older women.
Strengths & Limitations
The prospective 6-year follow-up and availability of multiple standardized clinical PPMs and PA data in a large, well-characterized cohort of women aged 65–79 y are clear strengths of this analysis; however self-reported recreational PA is limited by recall and desirability biases, which may lead to misclassification of activity levels.23 For example, in the present study, bias associated with self-reporting PA may explain the increase in activity levels over the 6 year follow-up among the sedentary PA group; conversely, the change in PA levels among the sedentary group may wrongly be attributed to a true increase, and may instead be caused by regression to the mean at the individual or group level. Although the WHI Physical Activity questionnaire was shown to have good reliability and validity,29 corroborating the reported declines in PA with objective measures is recommended to further relate, according to dose (intensity, frequency and duration), to functional status changes in older women. PA in this cohort was primarily in the form of walking or other recreational aerobic activity so the role of muscle-strengthening, stretching, and lower intensity aerobic exercise on PPMs is not clear. Further, relatively healthy post-menopausal women, potentially with higher functional status enrolled in the WHI clinical trials may limit how generalizable the results are to the general population (including men). Additionally, the study population consisted primarily of white women and results may therefore not be generalizable to individuals of other race and ethnic backgrounds. Finally, despite controlling for many potential variables, residual confounding may influence our observed associations between PA and PPMs.
Conclusion
In conclusion, improved physical function over 6 years, as assessed by physical performance measures of gait speed, chair stands and grip strength, is associated with higher initial physical activity (PA) levels in women aged 65–79, increases in physical activity over time, and with maintenance of even low PA levels, compared to sedentary behavior. These data support the national recommendations for older women to increase or maintain PA levels as they age.
Supplementary Material
Supplementary Table S1. Baseline characteristics across levels of physical activity: mean ± SD or n (%)
*P values were calculated using chi-squared tests for categorical variables and ANOVA for continuous variables.
Boldface indicates statistical significance (P<0.05)
Supplementary Table S2. Average and change in physical activity levels at each follow-up, by baseline physical activity category
aSedentary (<100); Low (100 to < 500); Moderate (500 to < 1200); High (≥1200)
Statistically significant at *P<0.05, †P<0.001 by Paired t-test
Acknowledgments
Funding: The WHI program was supported by the National Heart, Lung, and Blood Institute, National Institutes of Health, U.S. Department of Health and Human Services through contracts HHSN268201600018C, HHSN268201600001C, HHSN268201600002C, HHSN268201600003C, and HHSN268201600004C. The research presented in this paper is that of the authors and does not reflect the official policy of the NIH or NHLBI. DRL was supported by the NIH/NHLBI Cardiovascular Disease Prevention Fellowship (T32-HL007034).
The authors thank the WHI investigators and staff for their dedication, and the study participants for making the program possible. A listing of WHI investigators can be found at https://www.whi.org/researchers/Documents%20%20Write%20a%20Paper/WHI%20Investigator%20Short%20List.pdf.
Footnotes
This work was submitted and accepted for a poster presentation to the 2017 American Heart Association EPI/Lifestyle 2017 Scientific Sessions, to be presented by Dr. Deepika Laddu on March 9, 2017, in Portland, Oregon.
Conflicts of Interest: None of the authors on this manuscript have any conflicts of interest or financial disclosures to report.
Author Contributions: DRL analyzed the data, interpreted the results and wrote the manuscript. DRL, and MLS conceptualized the research question and designed the study. MLS, BCW, DOG, RB, and MJL provided consultation on methodology and helped with analysis interpretation. BCW performed the statistical data analysis, and contributed to the interpretation of results. DOG, RB, EG, AHS, SBG, MJL, BC, MSL, JAC, and CAT provided critical revisions to the manuscript. All authors approved the final version of the submitted and published manuscript and take responsibility for the integrity of the data and the accuracy of the data analysis. ICMJE criteria for authorship read and met: DRL, BCW, DOG, RB, EG, AHS, SBG, MJL, BC, MSL, JAC, CAT, MLS. Agree with manuscript results and conclusions: DRL, BCW, DOG, RB, EG, AHS, SBG, MJL, BC, MSL, JAC, CAT, MLS.
Sponsors role: NONE
References
- 1.From the Centers for Disease Control and Prevention. Public health and aging: trends in aging--United States and worldwide. JAMA : the journal of the American Medical Association. 2003;289(11):1371–1373. [PubMed] [Google Scholar]
- 2.Stessman J, Hammerman-Rozenberg R, Cohen A, Ein-Mor E, Jacobs JM. Physical activity, function, and longevity among the very old. Archives of internal medicine. 2009;169(16):1476–1483. doi: 10.1001/archinternmed.2009.248. [DOI] [PubMed] [Google Scholar]
- 3.Motl RW, McAuley E. Physical activity, disability, and quality of life in older adults. Physical medicine and rehabilitation clinics of North America. 2010;21(2):299–308. doi: 10.1016/j.pmr.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 4.Keysor JJ. Does late-life physical activity or exercise prevent or minimize disablement? A critical review of the scientific evidence. American journal of preventive medicine. 2003;25(3 Suppl 2):129–136. doi: 10.1016/s0749-3797(03)00176-4. [DOI] [PubMed] [Google Scholar]
- 5.Li J, Siegrist J. Physical activity and risk of cardiovascular disease--a meta-analysis of prospective cohort studies. International journal of environmental research and public health. 2012;9(2):391–407. doi: 10.3390/ijerph9020391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Patel KV, Coppin AK, Manini TM, et al. Midlife physical activity and mobility in older age: The InCHIANTI study. American journal of preventive medicine. 2006;31(3):217–224. doi: 10.1016/j.amepre.2006.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xue QL, Bandeen-Roche K, Mielenz TJ, et al. Patterns of 12-year change in physical activity levels in community-dwelling older women: can modest levels of physical activity help older women live longer? American journal of epidemiology. 2012;176(6):534–543. doi: 10.1093/aje/kws125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brach JS, FitzGerald S, Newman AB, et al. Physical activity and functional status in community-dwelling older women: a 14-year prospective study. Archives of internal medicine. 2003;163(21):2565–2571. doi: 10.1001/archinte.163.21.2565. [DOI] [PubMed] [Google Scholar]
- 9.Simonsick EM, Guralnik JM, Volpato S, Balfour J, Fried LP. Just get out the door! Importance of walking outside the home for maintaining mobility: findings from the women's health and aging study. Journal of the American Geriatrics Society. 2005;53(2):198–203. doi: 10.1111/j.1532-5415.2005.53103.x. [DOI] [PubMed] [Google Scholar]
- 10.Brach JS, Simonsick EM, Kritchevsky S, Yaffe K, Newman AB. The association between physical function and lifestyle activity and exercise in the health, aging and body composition study. Journal of the American Geriatrics Society. 2004;52(4):502–509. doi: 10.1111/j.1532-5415.2004.52154.x. [DOI] [PubMed] [Google Scholar]
- 11.Chale-Rush A, Guralnik JM, Walkup MP, et al. Relationship between physical functioning and physical activity in the lifestyle interventions and independence for elders pilot. Journal of the American Geriatrics Society. 2010;58(10):1918–1924. doi: 10.1111/j.1532-5415.2010.03008.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Design of the Women's Health Initiative clinical trial and observational study. The Women's Health Initiative Study Group. Controlled clinical trials. 1998;19(1):61–109. doi: 10.1016/s0197-2456(97)00078-0. [DOI] [PubMed] [Google Scholar]
- 13.Anderson GL, Manson J, Wallace R, et al. Implementation of the Women's Health Initiative study design. Annals of epidemiology. 2003;13(9 Suppl):S5–17. doi: 10.1016/s1047-2797(03)00043-7. [DOI] [PubMed] [Google Scholar]
- 14.Sims ST, Kubo J, Desai M, et al. Changes in physical activity and body composition in postmenopausal women over time. Medicine and science in sports and exercise. 2013;45(8):1486–1492. doi: 10.1249/MSS.0b013e31828af8bd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sims ST, Larson JC, Lamonte MJ, et al. Physical activity and body mass: changes in younger versus older postmenopausal women. Medicine and science in sports and exercise. 2012;44(1):89–97. doi: 10.1249/MSS.0b013e318227f906. [DOI] [PubMed] [Google Scholar]
- 16.Ainsworth BE, Haskell WL, Leon AS, et al. Compendium of physical activities: classification of energy costs of human physical activities. Medicine and science in sports and exercise. 1993;25(1):71–80. doi: 10.1249/00005768-199301000-00011. [DOI] [PubMed] [Google Scholar]
- 17.Ainsworth BE, Haskell WL, Herrmann SD, et al. 2011 Compendium of Physical Activities: a second update of codes and MET values. Medicine and science in sports and exercise. 2011;43(8):1575–1581. doi: 10.1249/MSS.0b013e31821ece12. [DOI] [PubMed] [Google Scholar]
- 18.2008 Physical Activity Guidelines for Americans. J Cardiovasc Nurs. 2009;24(1):2–3. [Google Scholar]
- 19.Onder G, Penninx BW, Lapuerta P, et al. Change in physical performance over time in older women: the Women's Health and Aging Study. J Gerontol A Biol Sci Med Sci. 2002;57(5):M289–293. doi: 10.1093/gerona/57.5.m289. [DOI] [PubMed] [Google Scholar]
- 20.Guralnik JM, Ferrucci L, Simonsick EM, Salive ME, Wallace RB. Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability. N Engl J Med. 1995;332(9):556–561. doi: 10.1056/NEJM199503023320902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Medical care. 1992;30(6):473–483. [PubMed] [Google Scholar]
- 22.Atkinson HH, Rapp SR, Williamson JD, et al. The relationship between cognitive function and physical performance in older women: results from the women's health initiative memory study. J Gerontol A Biol Sci Med Sci. 2010;65(3):300–306. doi: 10.1093/gerona/glp149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Keadle SK, McKinnon R, Graubard BI, Troiano RP. Prevalence and trends in physical activity among older adults in the United States: A comparison across three national surveys. Preventive medicine. 2016;89:37–43. doi: 10.1016/j.ypmed.2016.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Martin KR, Koster A, Murphy RA, et al. Changes in daily activity patterns with age in U.S. men and women: National Health and Nutrition Examination Survey 2003–04 and 2005–06. Journal of the American Geriatrics Society. 2014;62(7):1263–1271. doi: 10.1111/jgs.12893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Perera S, Mody SH, Woodman RC, Studenski SA. Meaningful change and responsiveness in common physical performance measures in older adults. Journal of the American Geriatrics Society. 2006;54(5):743–749. doi: 10.1111/j.1532-5415.2006.00701.x. [DOI] [PubMed] [Google Scholar]
- 26.Whitney SL, Wrisley DM, Marchetti GF, Gee MA, Redfern MS, Furman JM. Clinical measurement of sit-to-stand performance in people with balance disorders: validity of data for the Five-Times-Sit-to-Stand Test. Physical therapy. 2005;85(10):1034–1045. [PubMed] [Google Scholar]
- 27.Leong DP, Teo KK, Rangarajan S, et al. Prognostic value of grip strength: findings from the Prospective Urban Rural Epidemiology (PURE) study. Lancet. 2015;386(9990):266–273. doi: 10.1016/S0140-6736(14)62000-6. [DOI] [PubMed] [Google Scholar]
- 28.Kuh D, Karunananthan S, Bergman H, Cooper R. A life-course approach to healthy ageing: maintaining physical capability. The Proceedings of the Nutrition Society. 2014;73(2):237–248. doi: 10.1017/S0029665113003923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Meyer AM, Evenson KR, Morimoto L, Siscovick D, White E. Test-retest reliability of the Women's Health Initiative physical activity questionnaire. Medicine and science in sports and exercise. 2009;41(3):530–538. doi: 10.1249/MSS.0b013e31818ace55. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table S1. Baseline characteristics across levels of physical activity: mean ± SD or n (%)
*P values were calculated using chi-squared tests for categorical variables and ANOVA for continuous variables.
Boldface indicates statistical significance (P<0.05)
Supplementary Table S2. Average and change in physical activity levels at each follow-up, by baseline physical activity category
aSedentary (<100); Low (100 to < 500); Moderate (500 to < 1200); High (≥1200)
Statistically significant at *P<0.05, †P<0.001 by Paired t-test