Abstract
In the study of cell penetrating and membrane translocating peptides, a fundamental question occurs as to the contribution arising from fundamental peptide-membrane interactions, relative to the contribution arising from the biology and energy of the cell. The latter contribution occurs in the form of endocytosis and subsequent events. A commonly used approach to begin addressing these mechanistic questions is to measure the degree to which peptides can interact with, and physically disrupt, the integrity of synthetic lipid bilayers. Here we describe a set of experimental methods that can be used to measure the potency, kinetics, transience, and the effective size of peptide-induced membrane disruption.
Keywords: Vesicle leakage, bilayer permeabilization, pore formation, leakage assay, terbium, ANTS, DPX, transient leakage, pore size
1. Introduction
Cell penetrating peptides (CPPs), including membrane translocating peptides (MTPs), have shown significant promise in the laboratory over the last couple of decades (1-9). There are examples of peptide-dependent in vitro delivery of small molecules, peptides, proteins, oligonucleotides and nanoparticles. CPPs and MTPs, by definition, enable the movement of polar molecules across membrane barriers, but there are many possible mechanisms by which this can happen. Some are active, driven by cellular processes such as endocytosis, and some are passive, driven directly by the physical chemistry of peptide-membrane interactions. Understanding the contribution of these two possible mechanisms is the key to improving and refining delivery strategies.
To assess the contribution of peptide-membrane interactions to the biological activity of CPPs and MTPs, cell membranes can be modeled by synthetic lipid vesicles. While synthetic bilayers are not exact mimics of cell membranes, there is much overlap in the physical chemistry of peptide interactions with synthetic and natural membranes. Thus, synthetic bilayers can be used as a simple and convenient model system with which to explore the fundamental interactions, and to explore the effects of peptides on membrane integrity. There are many experimental methods that have been developed to explore these effects. The information provided by such methods provide important information about the potential mechanisms of action of cell penetrating and membrane translocating peptides.
Here we will describe a set of protocols that can be used to probe the effect of cell penetrating and membrane translocating peptides on the integrity of lipid bilayer membranes, both as a test of potential cytotoxicity and as a way of inferring mechanism of action. First we will describe the ANTS/DPX assay (10,11) and the Tb3+/DPA assay (12) that both can be used to measure the leakage of small molecule probes from lipid vesicles. Second, we will describe the equilibrium pore assay (13) that can be used to measure probe leakage and simultaneously assess whether the leakage pathway in vesicles is a transient or an equilibrium phenomenon. This is an important clue to mechanism. Third, we will describe a dextran escape assay (14) that can be used to assess whether the peptide-induced pathways through lipid bilayers can enable the passage of macromolecules. The molecules used in these assays are shown in Figure 1.
Figure 1.
The probe compounds used in the vesicle leakage assays described in this chapter. Counterions are shown when they contribute to osmolarity. Formula weights (FW) are for the probe without counterions to indicate the size of the molecule that must pass through the membrane to report on permeabilization. A: ANTS/DPX assay; B: Tb3+/DPA assay; C: The second part of the equilibrium pore assay, which also uses the Tb3+/DPA assay; D: Dextran release assay.
Some researchers have modeled membrane permeabilizing peptides as membrane-spanning, proteinacious pores, sometimes drawing them as rings of parallel helices in a barrel-stave pattern across the membrane. However, experimental support for the existence of such explicit peptide pores is very rare (15-18). Even the archetypal “pore-forming peptide”, melittin, cannot be described accurately with a proteinaceous pore model under many conditions (15,19,20). As a result, alternate, overlapping mechanistic models have been proposed that describe most peptide-induced membrane leakage as cooperative destabilization of membrane integrity, perhaps in the vicinity of peptide-rich domain in the bilayer (18,21). Even for the macromolecule-sized poration observed for a few peptides (14), long-lived, “barrel-stave” pores seem improbable as that structure would require a stable, continuous “ring” of 20 or more peptides to enable macromolecule passage through the bilayer. Instead, “pores” formed by peptides, including CPPs will likely be caused by dynamic, cooperative destabilization of membrane structure within peptide-rich membrane domains, perhaps similar to the “toroidal pores” described by some researchers (22-24).
To fully communicate the effect that a peptide has on the integrity of a lipid bilayer, four properties must be described: Potency, kinetics, transience and pore size. The potency of a peptide describes the ability of a peptide to disrupt a lipid bilayer, on a peptide per lipid basis. Potency is best described using the ratio of bound peptide to total lipid (Pbound:L). The overall sample P:L sets only an upper limit on the possible Pbound:L. In order to determine Pbound, membrane binding must be measured. Fractional peptide binding can be measured by equilibrium dialysis (25), fluorescence titration (26), circular dichroism titration, filter binding, and other methods. Potency is important to note because almost any membrane-interacting peptide will disrupt bilayers at a high enough Pbound:L. (P:L ≥1:50), a concentration range that is likely not relevant to the biological activity of CPPs and MTPs. The most informative way to express potency is to record the Pbound:lipid that causes 50% effect, or PL50. This parameter ranges from ≥ 1, for peptides that have no effect on bilayers, to ≤ 1:2000, for the most potent membrane-disrupting peptides known (13,27). Real examples of potency measurements are shown in Figure 2. Note that all the curves have a similar shape, an observation that holds true across many studies.
Figure 2.
Various example leakage potency curves for membrane-active peptides. These data are from the author's laboratory and were selected from multiple papers and unpublished experiments. They were not all collected under the same conditions but are plotted together and scaled to 0.5 mM lipid to serve as examples of the range of possible potencies. A few peptides can permeabilize vesicles efficiently at P:L ≤ 1:1000. However most membrane active peptides cause permeabilization only at P:L ≥ 1:100. Under some conditions, cell penetrating peptides permeabilize lipid vesicles, usually in the range P:L ≥ 1:100.
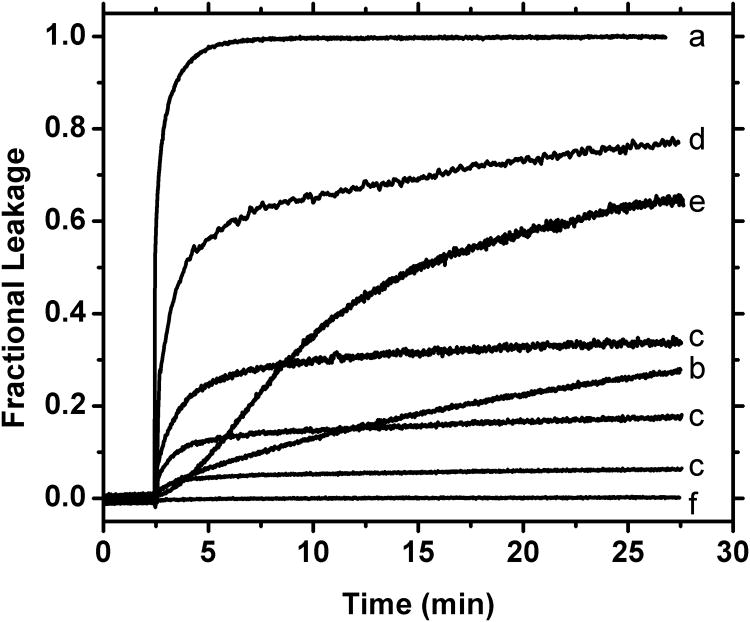
The kinetics of leakage describe the rate at which leakage occurs and the rate at which it stops. Rate measurements provide important information about the permeabilization mechanism. Peptide-induced leakage of solutes from lipid vesicles can range from almost instantaneous (t1/2 < 30 seconds) to very slow with T1/2 > 10 hours. However most peptide-induced leakage occurs within 2-30 minutes after peptide addition. Various real examples of leakage time courses are shown in Figure 3, demonstrating the range of likely behaviors.
Figure 3.
Various leakage time course curves from the author's laboratory. These data, which were selected from multiple papers and unpublished experiments, show a variety of possible kinetic behaviors. They are not all collected under the same conditions but are plotted together here to serve as examples of the range of possible kinetic behaviors. Behaviors include (a) rapid total permeabilization, (b) slow total permeabilization, (c) transient burst of leakage with zero background leakage (three examples are shown for three P:L values), (d) transient burst with slow background leakage, (e) delayed leakage, and (f) no leakage. Leakage kinetics provide information about possible mechanisms of membrane permeabilization by peptides. Note that curves c, d and e can only be explained by transient permeabilization.
The transience of permeabilization describes the lifetime of the disruption of bilayer integrity. Surprisingly, the majority of published examples of peptide-induced membrane permeabilization occur through transient, non-equilibrium processes (13,28). Leakage occurs only in the minutes immediately after peptide addition. The system then relaxes to a state where leakage slows or stops completely despite the continues presence of the peptides in the bilayer. Transient leakage cannot be correctly modeled as an equilibrium state of the membrane. Even the archetypal pore forming peptide melittin causes transient, non-equilibrium permeabilization at moderate peptide concentrations (Pbound:L ≤ 1:100). The reason for transient leakage has not been definitively proven. The leading hypothesis is that the initial binding of peptide to the surface of the bilayer causes an imbalance of mass, charge or surface tension, which is dissipated by the stochastic, transient failure of the bilayer structure. When the asymmetry of peptide distribution has been relieved, the permeabilization no longer occurs. Transient membrane disruption means that simulations and structural modelling based on equilibrium phenomena are unlikely to reveal true mechanistic details.
The “pore” size allows a description of the size of the disruption of bilayer integrity. Peptide-induced release of molecules from lipid vesicles is generally probed using small fluorescent probes of a few hundred Daltons. But important information on the characteristics of the membrane disruption can also be obtained by examining the dependence of membrane permeabilization on the size of the probe. Some membrane permeabilizing peptides, at high concentration (Pbound:L ≥ 1:50), disrupt vesicles catastrophically (19,29,30), such that all entrapped probes escape equally well, independent of size. Other peptides show a distinct size dependence for leakage, indicating a more well defined pathway for solute escape (31). Finally, some peptides do not release macromolecules at all, indicating that only small pores are formed. Information on pore size provides important clues to the mechanism of peptide-induced disruption of lipid bilayer membranes.
In the sections that follow, methods are described for characterizing the peptide-induced disruption of lipid bilayer membranes with respect to the four parameters described above. Specifically we describe two methods for assaying the potency and kinetics of probe release, a method for measuring both the potency and the transience of membrane disruption in the same vesicles, and a method for measuring the release of macromolecules from lipid vesicles, which can be used to probe the size of the “pore” in the membrane.
2. Materials
2.1 Lipids
POPC (1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine) chloroform solution.
POPG (1-palmitoyl-2-oleoyl-sn-glycero-3-phosphoglycerol) chloroform solution (optional).
POPE (1-palmitoyl-2-oleoyl-sn-glycero-3-phosphoethanolamine) chloroform solution (optional).
POPS (1-palmitoyl-2-oleoyl-sn-glycero-3-phosphoserine), chloroform solution (optional).
Cholesterol, chloroform solution (optional).
Sphingomyelin, chloroform solution (optional).
2.2 ANTS/DPX Leakage Assay
ANTS (8-aminonaphthalene-1,3,6-trisulfonic acid disodium salt), solid powder.
DPX (p-xylene-bis-pyridinium bromide), solid powder.
Lipids above dissolved in chloroform.
Vesicle preparation buffer: 10 mM phosphate or other buffer compound. No added NaCl (See Note 1). Any pH between 3.5 and 8.5.
Vesicle elution buffer: 10 mM phosphate or other buffer compound + 40 mM NaCl. (See Note 1)
10% v/v Reduced Triton X-100 in water (See Note 2).
Lipid extruder, with drain discs and 0.1 μm pore size Nuclepore polycarbonate filters.
Sephadex G200 gel filtration resin or 5 ml 100k MWCO centrifugal concentrator filter units.
Glass column for gel filtration of 25 × 1 cm.
2.3 Tb3+/DPA Leakage Assay
1. TbCl3, (Terbium(III) chloride, hexahydrate), solid powder.
2. DPA (Dipicolinic acid), solid powder.
3. Sodium citrate, solid powder.
4. Lipids above dissolved in chloroform.
5. Vesicle preparation buffer: 10 mM TES, pH 7.2, 50 mM sodium citrate (no added NaCl) (See Note 3)(See Note 4).
6. Vesicle elution buffer: 10 mM TES pH 7.2 +150 mM NaCl (See Note 4).
7. Assay buffer: Vesicle elution buffer + 150 μM DPA.
7. 10% v/v Reduced Triton X-100 (See Note 2)
8. Lipid extruder, with drain discs and 0.1 μm pore size Nuclepore polycarbonate filters.
9. Sephadex G200 gel filtration resin or 5 ml 100k MWCO centrifugal concentrator filter units.
10. Glass column for gel filtration of 25 × 1 cm.
2.4 The Equilibrium Pore Assay
All materials for the Tb3+/DPA assay, above, plus the following:
POPE-NBD (1,2-dioleoyl-sn-glycero-3-phosphoethanolamine-N-(7-nitro-2-1,3-benzoxadiazol-4-yl) (ammonium salt)), chloroform solution.
Sodium dithionite (sodium hydrosulfite) solid powder (See Note 5).
Sodium phosphate buffer, 1.0 M Na2PO4, pH 10.0.
2.5 Synthesis of TAMRA-biotin-dextran
Tentagel-S-NH2 solid phase peptide synthesis resin.
10,000, 40,000 or 70,000 Da amino dextran with at least 4, 10 or 12 amino groups per dextran molecule, respectively, solid powder.
TAMRA-SE (5-and 6-TAMRA (mixed isomers) succinamidyl ester), solid powder.
Biotin-SE (biotin succinamidyl ester), solid powder.
50 mM KHPO4 buffer, pH 8.4.
2.6 Dextran Release Assay
TAMRA-biotin-dextran, solid powder.
Alexafluor488-Streptavidin, solution.
Biotin, solid powder.
Lipids above dissolved in chloroform.
Vesicle preparation buffer of user's choice.
Sample buffer: Vesicle preparation buffer + 20 nM AlexaFluor488-streptavidin.
10% v/v reduced triton X-100 in water.
Sephadex G200 gel filtration resin or 5 ml 100k MWCO centrifugal concentrator filter units.
Glass column, 25 × 1 cm.
3. Methods
All the methods described below rely on the preparation of unilamellar (single bilayer) lipid vesicles, with a probe molecule, or probe molecules, entrapped inside. The probe molecules are removed from the external solution after vesicle preparation as described such that the release of entrapped probes can be measured by fluorescence. The so-called “large” unilamellar vesicles (LUV) of 0.1 μm diameter, made by extrusion (32), are the most commonly used synthetic vesicle system.
3.1 Preparation of Large Unilamellar Vesicles with Entrapped Probes
Mix a total of 250 μmoles of lipids in chloroform in an appropriate glass vessel. The lipid composition is defined by the user's experimental questions. Dry off the bulk of the chloroform under a stream of nitrogen or in a rotary evaporator. Place the vessel in high vacuum for at least 4 hours to evaporate any traces of remaining solvent.
Add 5 ml of the appropriate buffer containing the probes to be entrapped (see below). This will make a solution of 50 mM lipids (See Note 6). Gently warm and swirl the buffer until the dried lipid film is completely suspended. (See Note 7).
To properly entrap the solutes and buffer ions within the lipid bilayers, repeatedly freeze the lipid suspension in liquid nitrogen, or in a dry ice/ethanol bath, and thaw the suspension in a warm (50°C) water bath. Repeat this 10 times for small molecule probes, and 20 times for macromolecule probes.
Repeatedly extrude the lipid suspension through two stacked Nuclepore polycarbonate membranes, with 0.1 μm diameter pores, placed on top of a polymer mesh drain disc.
Separate the vesicles with their entrapped contents from external probes using gel filtration chromatography. Prepare a 25 × 1 cm low pressure, clear glass column of Sephadex G200. Slowly add vesicles and continue buffer flow through the column. Monitor the movement of vesicles and probes through the column visually, or with a handheld UV light. The leading vesicle band will be opalescent. The trailing external probe band (if it is ANTS or TAMRA-biotin-dextran) band will be visibly fluorescent when illuminated with long wave UV light. The trailing Tb3+/citrate band will not be visible until DPA is added. Manually collect the fractions containing highest concentration of lipid (See Note 8).
As an alternative method of separating external probes from entrapped probes, 5 mL 100KDa MWCO centrifugal sample concentrators can be used. Concentrate the vesicle samples, replace the lost buffer with vesicle elution buffer, and resuspend the vesicles. Repeat at least ten times. Save the individual flow through fractions after repeat number 5 for analysis. (See Note 1)(See Note 4). Assay the flow through fractions for external probe concentration. Measure entrapped probe by lysing an aliquot of vesicles with 0.2% v/v reduced Triton-X-100. (See Note 9). Stop replacing buffer when the external probe concentration is less than 5% of the entrapped probe concentration in the vesicles.
Measure lipid concentration in the final solution using the method of Bartlett (33) or Stewart (34).
3.2 ANTS/DPX Leakage Methods
Prepare a dried lipid sample as described in section 3.1.
Prepare a solution of 20 mM ANTS and 6 mM DPX in vesicle preparation buffer (See Note 1).
Suspend the lipids in the ANTS/DPX buffer as described in Section 3.1
Freeze-thaw and extrude lipid suspension as described in Section 3.1 to make large unilamellar vesicles (LUV).
Separate vesicles from external ANTS/DPX using gel filtration (section 3.1.5) or centrifugal filtration (section 3.1.6). The external solution should be vesicle elution buffer (See Note 1)(See Note 10).
To measure the leakage of ANTS/DPX, dilute vesicles to a concentration of 0.5 mM lipid. Add peptide to individual samples at concentrations from 0.5 μM (P:L = 1:1000) to 50 μM (P:L=1:10). (See Note 11)
Monitor ANTS fluorescence using a fluorimeter (excitation = 350 nm; emission = 530 nm) or a fluorescence plate reader (excitation filter wavelength/bandpass = 340/11; emission 530/25; Do not use a dichroic mirror. Make sure there is no default UV filter in the excitation path). (See Note 12).
When leakage has stopped or slowed significantly (generally 10-60 minutes) measure the final ANTS fluorescence. Then add 0.2% v/v final concentration of reduced Triton-X100 to lyse the vesicles and measure fluorescence corresponding to 100% ANTS.
Calculate fractional leakage as (Fsample-Finitial)/(Flysed-Finitial). (See Note 13).
3.3 Tb3+/DPA Leakage Methods
Prepare a dried lipid sample as described in section 3.1.
Prepare a solution of 25 mM TbCl3 in vesicle preparation buffer. (See Note 3)(See Note 4).
Suspend the lipids in the Tb3+/citrate vesicle preparation buffer.
Freeze-thaw and extrude the lipid suspension as described in Section 3.1 to make large unilamellar vesicles (LUV).
Separate vesicles from external Tb3+/citrate using gel filtration (section 3.1.5) or centrifugal filtration (section 3.1.6). The external solution in either case should be vesicle elution buffer (See Note 4)(See Note 10).
To measure leakage of Tb3+/citrate and DPA, dilute vesicles to a concentration of 0.5 mM in assay buffer which contains 150 μM DPA. Add peptide to individual samples at concentrations from 0.5 μM (P:L = 1:1000) to 50 μM (P:L=1:10) (See Note 11).
Monitor Tb3+/DPA phosphorescence using a fluorimeter (excitation = 270 nm; emission = 490 nm or 545 nm) or a plate reader (excitation filter wavelength/bandpass 270/15; emission 540/30; use no dichroic mirror or UV filters). (See Note 12).
When leakage has stopped or slowed significantly (typically 10-60 minutes) measure final Tb3+/DPA phosphorescence. Then add 1% v/v of 10% reduced Triton-X100 (final concentration 0.2% v/v) to lyse the vesicles and measure 100% Tb3+/DPA phosphorescence.
Calculate fractional leakage as (Fsample-Finitial)/(Flysed-Finitial).
3.4 The Equilibrium Pore Assay Methods
In the equilibrium pore assay, an additional measurement is added to the Tb3+/DPA assay described above in section 3.3.
Prepare Tb3+ containing vesicles exactly as described above except replace 1 mol% of PC lipid with NBD-POPE lipid.
Each day, prepare a fresh solution of 0.6 M sodium dithionite in 1.0 M phosphate buffer, pH 10.
Measure Tb3+/DPA leakage exactly as described above, except that the experimental samples are not lysed with Triton X-100. A separate sample is lysed to determine the Tb3+/DPA intensity for 100% leakage.
Allow transient processes to reach completion in experimental samples by equilibrating for at least 4 hours. Overnight equilibration is also possible.
Add 5% v/v of the fresh dithionite solution to the experimental samples.
Measure the NBD intensity loss in samples as a function of time for 60 minutes after adding dithionite. Also measure NBD loss in intact vesicles and in Triton X-100 lysed vesicles. The two control samples provide measurements of the rate of NBD fluorescence loss when it is exposed to the external space, and when some if it is not exposed. (See Note 14).
Determine the %NBD that is quenchable from the breakpoint between rapid quenching and slow quenching. In intact, control vesicles about 55% of the NBD lipids are exposed on the outer monolayer and are rapidly quenched by dithionite. The remainder of the NBD lipids are on the inner monolayer, not exposed to dithionite. They are quenched very slowly as small amounts of dithionite equilibrate across the bilayer. In Triton-lysed vesicles 100% of the NBD is effectively “exposed” and is rapidly quenched. Peptides that cause transient pores will not (at equilibrium) substantially increase the fraction of NBD that is accessible. Peptides that cause equilibrium pores, allow for most of the NBD to be rapidly quenched at equilibrium because equilibrium pores enable uninhibited entry of dithionite into the vesicle interior.
3.5 Synthesis of TAMRA-biotin-dextran
Dissolve 50 mg dextran in 2 ml KHPO4 buffer, pH 8.4.
Dissolve 10 mg TAMRA-SE in 0.4 ml buffer.
Dissolve 2 mg of biotin-SE in 0.4 ml buffer.
Mix the two labels together first, and then add them to the dextran
React at room temperature for 4 hours with gentle shaking.
After 4 hours, add 0.3 g of the peptide synthesis resin to react with most of the remaining unreacted labels. Mix well for 30 minutes, spin down the resin and remove the supernate.
Split the supernatant into two 15 ml tubes, with ∼1.5 ml of the reaction mixture in each tube.
Add 12 ml of cold methanol to each tube. Mix and incubate at 4°C for 15 min.
Pellet the large amount of colored, precipitated dextran by centrifugation. Discard the supernate.
Wash pellet as above 5-10 times with 10 ml cold methanol. Stop washing when the supernatant is colorless.
Dry the methanol from the dextran pellet under vacuum, and then dissolve the dextran in distilled water and lyophilize.
If blockage of all available amino groups on the dextran is critical, dissolve the dual labeled dextran at 100 mg/ml in water and add 5% diisopropylethylamine + 5% acetic anhydride. React for 30 minutes to acetylate amino groups. Precipitate the dextran with cold methanol as above, and wash at least 5 times with cold methanol. Dry the methanol off, dissolve the dextran in distilled water, and lyophilize.
3.6 Dextran Escape Methods
Prepare a dried lipid sample as described in section 3.1.
Prepare a solution of 1 mg/ml TAMRA-biotin-dextran in buffer. (See Note 15).
Suspend the lipids in the TAMRA-biotin-dextran buffer.
Freeze-thaw the lipid suspension 20 times and extrude as described in Section 3.1 to make large unilamellar vesicles (LUV).
Separate vesicles from external dextran using gel filtration (section 3.1.5) or centrifugal filtration (section 3.1.6).
To measure leakage of dextran, dilute vesicles to a concentration of 0.5 mM into assay buffer which contains 40 nM AlexaFluor488-Streptavidin (See Note 10). To individual samples, add peptide at concentrations from 0.5 μM (P:L = 1:1000) to 50 μM (P:L=1:10) (See Note 11).
Monitor AlexaFluor488 fluorescence using a fluorimeter (excitation = 488 nm; emission = 510 nm) or a fluorescence plate reader (excitation filter wavelength/bandpass 480/30; emission 520/30; 505 nm dichroic mirror) (See Note 12).
When dextran leakage has stopped or slowed significantly (typically 10-60 minutes) measure final AlexaFluor488 fluorescence. Then add 2% v/v of 10% reduced Triton-X100 (final concentration 0.2% v/v) to lyse the vesicles and measure AlexaFluor488 fluorescence corresponding to complete dextran release. (See Note 16)
Calculate fractional leakage as (Finitial-Fsample)/(Finitial-Flysed).
4.0 Notes
The osmolarity of the ANTS and DPX in the vesicle preparation buffer must equal the osmolarity of the NaCl in the vesicle elution buffer. Assuming osmotic coefficients of 1 then 3*[ANTS] + 3*[DPX] must equal 2*NaCl. In this protocol 20 mM ANTS and 6 mM DPX = ∼40 mM NaCl. The buffers recommended here have 0 NaCl in the vesicle preparation buffer and 40 mM in the vesicle elution buffer. However osmotic balance will be maintained in any buffer system in which the vesicle elution buffer has 40 mM more monovalent salt than the vesicle preparation buffer.
It is critical to use “reduced” Triton X-100 for all applications where fluorescence is being used. Ordinary Triton X-100 from scientific supply companies has a very large fluorescent background signal.
Terbium (III) is not very soluble in water unless it is chelated. Citrate is used to chelate the metal, and is readily displaced by DPA when DPA is present. Furthermore, Tb3+ cannot be used in the presence of phosphate, so an organic buffer such as TES or HEPES must be used.
The osmolarity of the TbCl3 and Na3Citrate in the vesicle preparation buffer must equal the osmolarity of the NaCl in the vesicle elution buffer. Assuming osmotic coefficients of 1, and ignoring the complex formation between Tb3+ and citrate, then 4*[TbCl3] + 4*[Na3Citrate] must equal 2*[NaCl]. In this protocol, 25 mM TbCl3 and 50 mM Na3citrate = 150 mM NaCl. The buffers recommended here have 0 NaCl in the vesicle preparation buffer and 150 mM NaCl in the vesicle elution buffer. However osmotic balance will be maintained in any buffer system in which the vesicle elution buffer has 150 mM more monovalent salt than the vesicle preparation buffer. In some work we have used 50 mM TbCl3 and 100 mM Na citrate in vesicle preparation and thus used 300 mM NaCl in vesicle elution.
Sodium dithionite should be handled and stored with special care as it can spontaneously heat and combust when exposed to air or atmospheric humidity.
The values for lipid amount and buffer volume are for a preparation of 5 ml of 50 mM lipid. Reduce both proportionally if smaller volumes are needed.
For some lipid compositions, as much as 15 minutes will be required to get the lipid film into suspension. Do not vortex lipids at any time, as this can lead to foaming.
It is important at this point to verify that a good separation between entrapped and external probe has been obtained. This can be measured by separating an aliquot of vesicles using a filter or spin concentrator and measuring the concentration of the probe (ANTS, Tb3+, TAMRA-biotin-dextran) in the flow-through. Compare the flow through concentration to the concentration in the retained vesicles (measured after lysing with 0.2% final v/v of Triton X-100). The external probe concentration should be less than 5% of the entrapped probe concentration. External ANTS can also be measured by titrating DPX into a sample of intact vesicles and measuring the quenching of ANTS fluorescence (10,11). External Tb3+ can be measured by adding DPA and measuring the increase in fluorescence. External TAMRA-biotin-dextran can be measured by adding external AlexaFluor488-streptavidin and measuring the quenching of AF488 relative to a parallel sample with no TAMRA-biotin-dextran.
Gel filtration and centrifugal concentration each have advantages and disadvantages. Gel filtration can be used to process large vesicle samples, and subjects the vesicles to fewer strong shear forces than centrifugation. However, gel filtration significantly dilutes the vesicles. Occasionally, due to imperfections in column packing, for example, separation of vesicles and external probe will be poor, leading to an entire failed batch of vesicles. Centrifugal buffer exchange is harsher on the vesicles due to physical shear forces of pelleting and resuspension, as well as temperatures of ∼45°C during long centrifugation. However we have found little or no evidence that vesicles are negatively affected by these conditions. This approach also allows the final vesicle concentration to be determined by the researcher at the final dilution step.
-
One can estimate the concentration of entrapped probe molecules in an experiment using trapped volume measurements. For POPC lipids, a typical fractional probe entrapment is about ∼0.0032 per mM lipid. Thus, if lipid concentration is expressed in mM units, then [Probe]Experiment = 0.0032*[Probe]Original *[Lipid(mM)Experiment]
As an example calculation, if we freeze-thaw and extrude vesicles in the presence of 20 mM ANTS, remove the external ANTS by gel filtration, and then perform an experiment at 0.5 mM lipid, we expect to have about 0.0032*20*0.5 = 0.032 mM, or 32 μM, ANTS in the experimental sample. Entrapment of 1 mg/ml TAMRA-biotin-dextran (25 μM) gives 40 nM dextran in an experiment with 0.5 mM lipid.
An LUV with an 0.1 μm diameter will have roughly 100,000 lipids, calculated by determining the sum of the inner and outer vesicle surface area and dividing by the area of a typical lipid molecule (∼70Å2). Thus at P:L = 1:1000 there are about 100 peptides per vesicle. At P:L = 1:50 there are about 2000 peptides per vesicle. Furthermore the entrapped volume of a single vesicle is about 4.5×10-19 liter. Thus, entrapment of 20 mM ANTS, for example, places about 5000 ANTS molecules inside each vesicle. Entrapment of 1 mg/ml TAMRA-biotin-dextran (40 μM) entraps about 10 dextrans in each vesicle. See Table 1.
One should always assess whether a peptide is causing large scale aggregation/fusion of vesicles by examining the sample for peptide-induced changes in light scattering and physical appearance (loss of opalescence, formation of visible peptide-vesicle aggregates, lipidic material falling out of suspension, etc.). Aggregation and fusion are extremely common artifacts in the cell penetrating peptide literature because, in a typical experiment, polycationic peptides are mixed with anionic bilayers at high concentration. This single artifact has likely caused decades of confusion about CPP mechanism of action because leakage of entrapped probes due to peptide-induced aggregation and fusion, while a common experimental observation, is likely to be completely unrelated to the biological activity of CPPs. To separate true membrane permeabilization from artifacts caused by aggregation and fusion, vesicle-vesicle interactions can be blocked by the addition of 4 mol % of PEG2000 lipids (35). The effect of PEG2000 lipids in peptide-induced aggregation of vesicles is shown in Figure 4. The effects of 4% PEG-lipids on membrane permeabilization that is not dependent on fusion, is small.
ANTS quenching is not a linear function of DPX concentration (10,11) as assumed in this commonly used equation. If leakage is “all-or-none” then the fluorescence will nonetheless be a linear function of leakage (10,11). However, if the leakage is “graded” then the fluorescence will not be linear with leakage. One can correct for this nonlinearity using a standard curve of ANTS intensity versus DPX concentration (10) but only if the mechanism is known. In the literature linearity is generally assumed because the mechanism, graded versus all-or-none, is unknown. In any case, the discrepancy is not significant as long the initial quenching is not more than about 80%. This is the reason the protocol described here uses DPX concentrations of only 6 mM.
Depending on the lipid composition and other factors, dithionite may slowly enter vesicles and quench internal NBD lipids. External NBD is quenched much more rapidly. The protocol is designed to detect the fraction of NBD that is quenched quickly, even when background quenching rate is relatively high.
In the dextran release assay, the osmolarity of the added dextran is so small that it can safely be ignored. Thus, the vesicle preparation buffer and vesicle elution buffer are identical.
To verify that the AlexaFluor488-streptavidin + TAMRA-biotin-dextran system is functioning as expected, one can carry out two parallel leakage experiments. One experiment in the presence, and one in the absence, of 100 μM free biotin. The biotin-blocked sample should show no effect of leakage while the biotin-free system should show 60-90% quenching of AF488-dex upon vesicle lysis. The exact amount of quenching depends on the ratio of the probes and the loading of TAMRA and biotin on the dextran.
Table 1.
The number of probe molecules entrapped per vesicle, and the number released per peptide (assuming 100% release) for typical ranges of concentrations. Notice that even relatively “potent” leakage only requires the release of a few probe molecules per peptide. Typical experimental conditions (e.g. 10 mM probe and P:L = 1:50) require that only 1-2 probe molecules are released per peptide over the course of a 20-60 minute incubation.
| Peptide Bound to Lipid Ratio | |||||||
|---|---|---|---|---|---|---|---|
| Probe Concentration (mM) | Probes per Vesicle | 1:1000 | 1:500 | 1:250 | 1:100 | 1:50 | 1:25 |
| 0.01 | 2.7 | 0.03 | >0.1 | >0.1 | >0.1 | >0.1 | >0.0 |
| 0.1 | 27 | 0.27 | 0.1 | 0.1 | >0.1 | >0.1 | >0.0 |
| 1 | 270 | 2.7 | 1.4 | 0.7 | 0.3 | 0.1 | >0.0 |
| 10 | 2,700 | 27 | 13.5 | 6.8 | 2.7 | 1.4 | 0.7 |
| 100 | 27,000 | 270 | 135 | 68 | 27 | 14 | 7 |
Figure 4.
Aggregation of anionic lipid vesicles by a cationic peptide can be blocked by PEG lipids. These vesicles contain 10 mol % anionic PG lipids + 90% PC lipids and are at 1 mM concentration. At 2 minutes, 20 μM of a membrane permeabilizing peptide with a net charge of +4 was added. In the absence of PEG-lipids, the sharp increase in light scattering shows that large scale aggregation occurs. Vesicles made with 1-3 mol % of PEG2000-lipids show sequentially less aggregation. Incorporation of ≥ 4 mol % PEG2000-lipids inhibits aggregation entirely.
References
- 1.Montrose K, Yang Y, Sun X, Wiles S, Krissansen GW. Xentry, a new class of cell-penetrating peptide uniquely equipped for delivery of drugs. Sci Rep. 2013;3:1661. doi: 10.1038/srep01661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dupont E, Prochiantz A, Joliot A. Penetratin story: an overview. Methods Mol Biol. 2011;683:21–29. doi: 10.1007/978-1-60761-919-2_2. [DOI] [PubMed] [Google Scholar]
- 3.Schmidt N, Mishra A, Lai GH, Wong GC. Arginine-rich cell-penetrating peptides. FEBS Lett. 2010;584:1806–1813. doi: 10.1016/j.febslet.2009.11.046. [DOI] [PubMed] [Google Scholar]
- 4.Chugh A, Eudes F, Shim YS. Cell-penetrating peptides: Nanocarrier for macromolecule delivery in living cells. IUBMB Life. 2010;62:183–193. doi: 10.1002/iub.297. [DOI] [PubMed] [Google Scholar]
- 5.Said HF, Saleh AF, Abes R, Gait MJ, Lebleu B. Cell penetrating peptides: overview and applications to the delivery of oligonucleotides. Cell Mol Life Sci. 2010;67:715–726. doi: 10.1007/s00018-009-0186-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Heitz F, Morris MC, Divita G. Twenty years of cell-penetrating peptides: from molecular mechanisms to therapeutics. Br J Pharmacol. 2009;157:195–206. doi: 10.1111/j.1476-5381.2009.00057.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.He J, Kauffman WB, Fuselier T, Naveen SK, Voss TG, Hristova K, Wimley WC. Direct Cytosolic Delivery of Polar Cargo to Cells by Spontaneous Membrane-translocating Peptides. J Biol Chem. 2013;288:29974–29986. doi: 10.1074/jbc.M113.488312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.He J, Hristova K, Wimley WC. A highly charged voltage sensor helix translocates spontaneously across membranes. Angew Chem Int Ed. 2012;51:7150–7153. doi: 10.1002/anie.201202741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marks JR, Placone J, Hristova K, Wimley WC. Spontaneous membrane-translocating peptides by orthogonal high-throughput screening. J Am Chem Soc. 2011;133:8995–9004. doi: 10.1021/ja2017416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ladokhin AS, Wimley WC, Hristova K, White SH. Mechanism of leakage of contents of membrane vesicles determined by fluorescence requenching. Methods Enzymol. 1997;278:474–486. doi: 10.1016/s0076-6879(97)78025-x. [DOI] [PubMed] [Google Scholar]
- 11.Ladokhin AS, Wimley WC, White SH. Leakage of membrane vesicle contents: Determination of mechanism using fluorescence requenching. Biophys J. 1995;69:1964–1971. doi: 10.1016/S0006-3495(95)80066-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rausch JM, Wimley WC. A high-throughput screen for identifying transmembrane pore-forming peptides. Anal Biochem. 2001;293:258–263. doi: 10.1006/abio.2001.5137. [DOI] [PubMed] [Google Scholar]
- 13.Krauson AJ, He J, Wimley WC. Determining the mechanism of membrane permeabilizing peptides: Identification of potent, equilibrium pore-formers. Biochim Biophys Acta. 2012;1818:1625–1632. doi: 10.1016/j.bbamem.2012.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wiedman G, Fuselier T, He J, Searson PC, Hristova K, Wimley WC. Highly efficient macromolecule-sized poration of lipid bilayers by a synthetically evolved peptide. J Am Chem Soc. 2014;136:4724–4731. doi: 10.1021/ja500462s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wimley WC. Describing the mechanism of antimicrobial peptide action with the interfacial activity model. ACS Chem Biol. 2010;5:905–917. doi: 10.1021/cb1001558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wimley WC, Hristova K. Antimicrobial Peptides: Successes, Challenges and Unanswered Questions. J Membr Biol. 2011;239:27–34. doi: 10.1007/s00232-011-9343-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bechinger B, Lohner K. Detergent-like actions of linear amphipathic cationic antimicrobial peptides. Biochim Biophys Acta. 2006;1758:1529–1539. doi: 10.1016/j.bbamem.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 18.Shai Y, Oren Z. From “carpet” mechanism to de-novo designed diastereomeric cell-selective antimicrobial peptides. Peptides. 2001;22:1629–1641. doi: 10.1016/s0196-9781(01)00498-3. [DOI] [PubMed] [Google Scholar]
- 19.Ladokhin AS, White SH. ‘Detergent-like’ permeabilization of anionic lipid vesicles by melittin. Biochim Biophys Acta. 2001;1514:253–260. doi: 10.1016/s0005-2736(01)00382-0. [DOI] [PubMed] [Google Scholar]
- 20.Wiedman G, Herman K, Searson P, Wimley WC, Hristova K. The electrical response of bilayers to the bee venom toxin melittin: evidence for transient bilayer permeabilization. Biochim Biophys Acta. 2013;1828:1357–1364. doi: 10.1016/j.bbamem.2013.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shai Y. Mode of action of membrane active antimicrobial peptides. Biopolymers. 2002;66:236–248. doi: 10.1002/bip.10260. [DOI] [PubMed] [Google Scholar]
- 22.Sengupta D, Leontiadou H, Mark AE, Marrink SJ. Toroidal pores formed by antimicrobial peptides show significant disorder. Biochim Biophys Acta. 2008;1778:2308–2317. doi: 10.1016/j.bbamem.2008.06.007. [DOI] [PubMed] [Google Scholar]
- 23.Ludtke SJ, He K, Heller WT, Harroun TA, Yang L, Huang HW. Membrane pores induced by magainin. Biochemistry. 1996;35:13723–13728. doi: 10.1021/bi9620621. [DOI] [PubMed] [Google Scholar]
- 24.Yang L, Harroun TA, Weiss TM, Ding L, Huang HW. Barrel-stave model or toroidal model? A case study on melittin pores. Biophys J. 2001;81:1475–1485. doi: 10.1016/S0006-3495(01)75802-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.White SH, Wimley WC, Ladokhin AS, Hristova K. Protein folding in membranes: Determining the energetics of peptide-bilayer interactions. Methods Enzymol. 1998;295:62–87. doi: 10.1016/s0076-6879(98)95035-2. [DOI] [PubMed] [Google Scholar]
- 26.Ladokhin AS, Jayasinghe S, White SH. How to measure and analyze tryptophan fluorescence in membranes properly, and why bother? Anal Biochem. 2000;285:235–245. doi: 10.1006/abio.2000.4773. [DOI] [PubMed] [Google Scholar]
- 27.Parente RA, Nir S, Szoka F. Mechanism of leakage of phospholipid vesicle contents induced by the peptide GALA. Biochemistry. 1990;29:8720–8728. doi: 10.1021/bi00489a031. [DOI] [PubMed] [Google Scholar]
- 28.Krauson AJ, He J, Wimley WC. Gain-of-Function Analogues of the Pore-Forming Peptide Melittin Selected by Orthogonal High-Throughput Screening. J Am Chem Soc. 2012;134:12732–12741. doi: 10.1021/ja3042004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hristova K, Selsted ME, White SH. Critical role of lipid composition in membrane permeabilization by rabbit neutrophil defensins. J Biol Chem. 1997;272:24224–24233. doi: 10.1074/jbc.272.39.24224. [DOI] [PubMed] [Google Scholar]
- 30.Goñi FM, Ostolaza H. E. coli a-hemolysin: A membrane-active protein toxin. Brazilian Journal of Medical and Biological Research. 1998;31:1019–1034. doi: 10.1590/s0100-879x1998000800002. [DOI] [PubMed] [Google Scholar]
- 31.Ladokhin AS, Selsted ME, White SH. Sizing membrane pores in lipid vesicles by leakage of co-encapsulated markers: Pore formation by melittin. Biophys J. 1997;72:1762–1766. doi: 10.1016/S0006-3495(97)78822-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nayar R, Hope MJ, Cullis PR. Generation of large unilamellar vesicles from long-chain saturated phosphatidylcholines by extrusion technique. Biochim Biophys Acta. 1989;986:200–206. [Google Scholar]
- 33.Bartlett GR. Phosphorus assay in column chromatography. J Biol Chem. 1959;234:466–468. [PubMed] [Google Scholar]
- 34.Stewart JC. Colorimetric determination of phospholipids with ammonium ferrothiocyanate. Anal Biochem. 1980;104:10–14. doi: 10.1016/0003-2697(80)90269-9. [DOI] [PubMed] [Google Scholar]
- 35.Hristova K, Kenworthy AK, McIntosh TJ. Effect of bilayer composition on the phase behavior of liposomal suspensions containing poly(ethylene glycol)-lipids. Macromolecules. 1995;28:7693–7699. [Google Scholar]