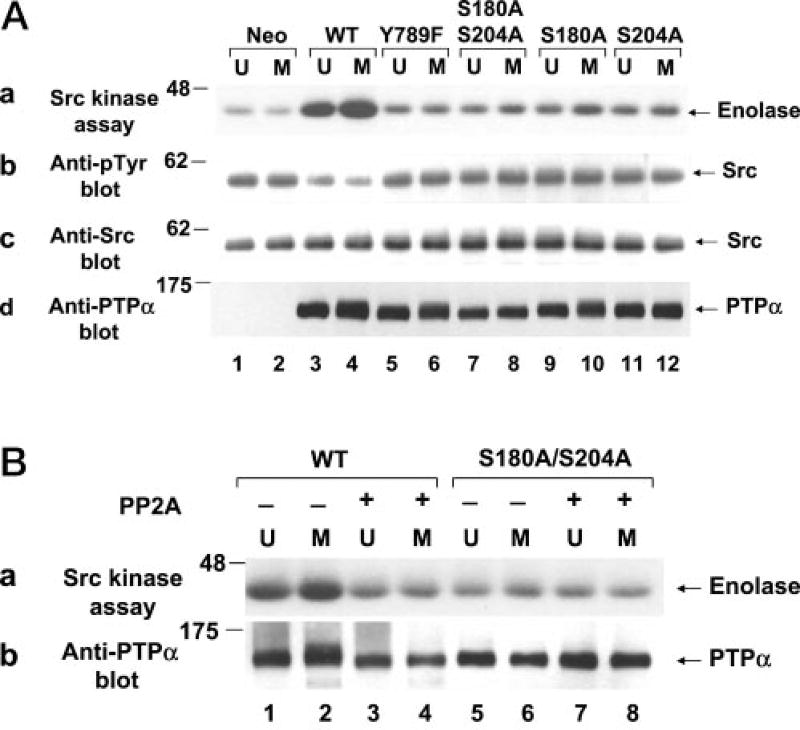
Fig. 2. Tyrosine dephosphorylation and activation of Src in vitro by WT and mutant PTPα from unsynchronized and mitotic cells.
A, Src that had been immunoprecipitated from NIH3T3-derived chicken Src overexpresser cells was incubated in phosphatase buffer with WT (lanes 3 and 4) or mutant (lanes 5–12) PTPα-HA that had been immunopurified from unsynchronized (U) or mitotic (M) PTPα overexpresser cells using anti-HA antibody or with mock-immunopurified protein from control cells that did not express any HA-tagged protein (lanes 1 and 2). The partially dephosphorylated Src immunoprecipitates were washed to remove PTPα and then incubated with enolase and [γ-32P]ATP in kinase buffer. Panel a, autoradiograph of [32P]enolase after the Src kinase assay; panel b, anti-Tyr(P) (Anti-pTyr) immunoblot of the Src immunoprecipitates after PTPα treatment; panel c, anti-Src immunoblot of the treated immunoprecipitates; panel d, anti-PTPα immunoblot of one-thirtieth of the PTPα used for the in vitro dephosphorylation reactions. B, the conditions were the same as described for A, except that immunopurified WT PTPα (lanes 1–4) and PTPα(S180A/S204A) (lanes 5–8) were treated (+; lanes 3, 4, 7, and 8) or not (−; lanes 1, 2, 5, and 6) with PP2A prior to incubation with Src. Panel a, autoradiograph of [32P]enolase after the Src kinase assay; panel b, anti-PTPα immunoblot of one-thirtieth of the PTPα used for the in vitro dephosphorylation reactions. SDS-PAGE was performed on 9% gels. The positions of molecular mass standards are indicated in kilodaltons.