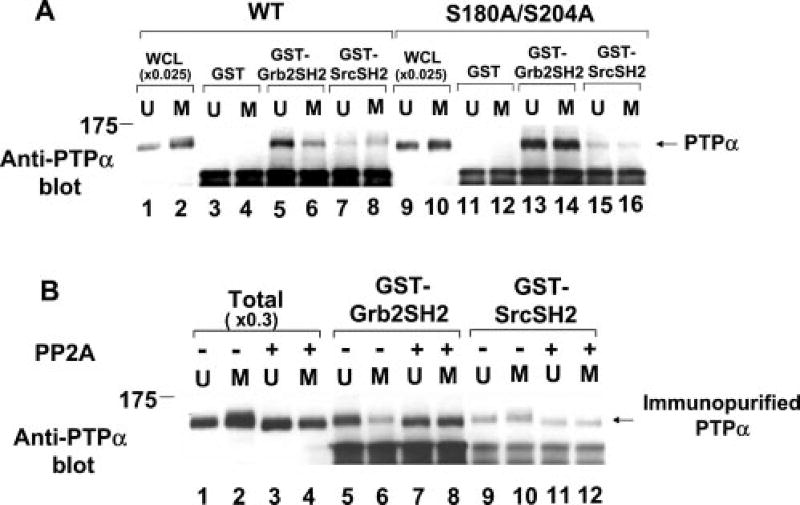
Fig. 7. Effects of Ser-to-Ala mutation and PP2A treatment on in vitro binding of PTPα from unsynchronized and mitotic cells to the Src and Grb2 SH2 domains.
A, lysates (containing 400 µg of total cell protein) from unsynchronized (U) or mitotic (M) WT PTPα-HA (lanes 3–8) or PTPα(S180A/S204A)-HA (lanes 11–16) overexpresser cells were affinity-precipitated by incubation with GST (lanes 3, 4, 11, and 12), a GST-Grb2 SH2 domain fusion protein (lanes 5, 6, 13, and 14), or a GST-Src SH2 domain fusion protein (lanes 7, 8, 15, and 16) bound to Sepharose beads. The washed beads were then analyzed by 9% SDS-PAGE and anti-PTPα immunoblotting. For comparison, lanes 1, 2, 9, and 10 contained 0.025 times the amount of complete whole cell lysate (WCL) used in the affinity precipitations. B, PTPα-HA was immunopurified from unsynchronized or mitotic overexpresser cell lysates (containing 1 mg of total cell protein), incubated with (+; lanes 7, 8, 11, and 12) or without (−; lanes 5, 6, 9, and 10) the serine/threonine phosphatase PP2A, and then affinity-precipitated by the GST-SH2 domain fusion proteins used for A. For comparison, lanes 1–4 (Total) contained 0.3 times the amount of immunopurified PTPα used in the affinity precipitations; these were also incubated with (lanes 3 and 4) or without (lanes 1 and 2) PP2A. The positions of molecular mass standards are indicated in kilodaltons.