Scheme 1:
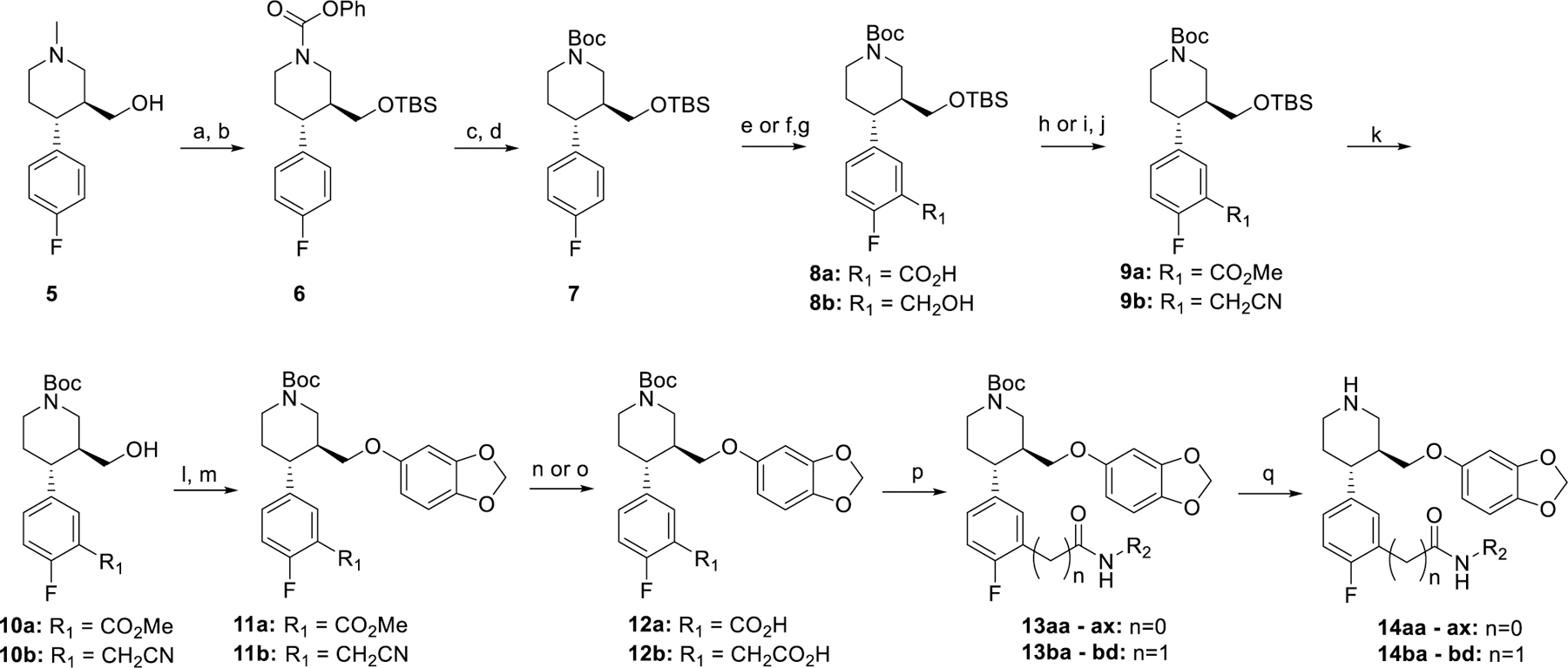
Synthesis of hybrid inhibitors 14aa – ax and 14ba - bd. Reagents and conditions. a) TBSCl, DIEA, imidazole, DCM, b) PCF, toluene, 110 ˚C then TEA, c) 8N NaOH, IPA, 80 ˚C, d) Boc2O, DIEA, DCM, e) TMEDA, sBuLi, THF, then CO2, f) TMEDA, sBuLi, THF, then DMF, g) NaBH4, THF, MeOH, h) TMS-diazomethane, MeOH/Toluene, i) Ms2O, DIEA, DCM, j) NaCN, DMSO, k) TBAF, THF, l) Ms2O, DIEA, DCM, m) NaH, 3,4-(methylenedioxy)phenol, DMF, 0 ˚C to 65 ˚C, n) 1N NaOH, MeOH, o) 50% NaOH, EtOH, 98 ˚C, p) DIEA, EDC, HOBt, R2NH2, q) HCl/dioxanes
