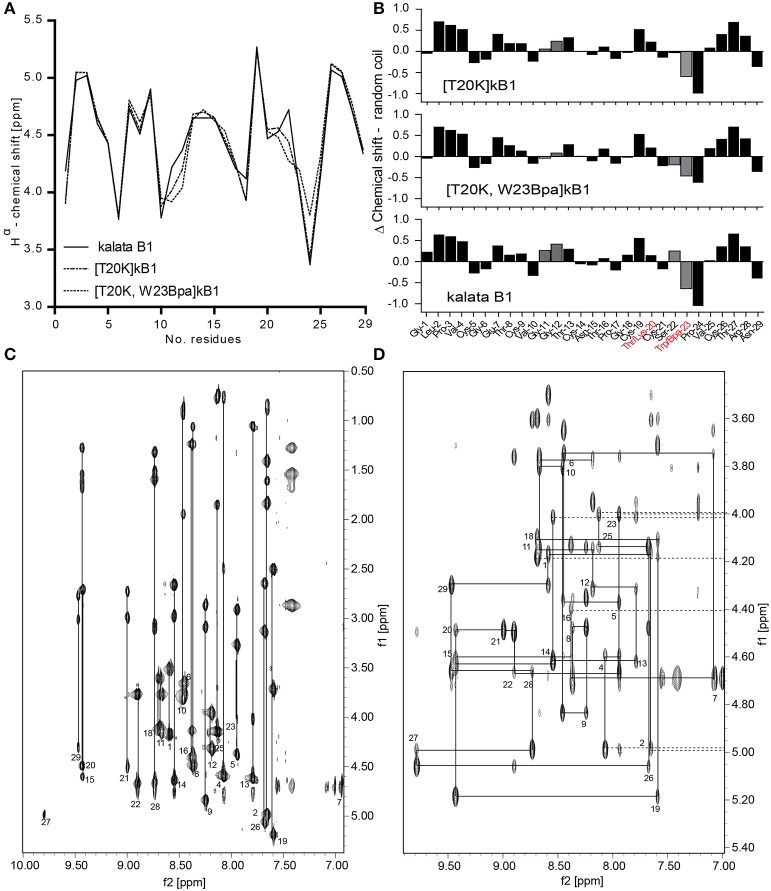
Figure 2.
Structural integrity of the circular peptide probe. (A) Superimposed Hα-chemical shifts of kalata B1 (kB1), [T20K]kB1, and [T20K,W23Bpa]kB1 are shown. (B) Secondary structure elucidation of the cyclotide probe [T20K,W23Bpa]kB1 in comparison to endogenous kB1 and the reference mutant [T20K]kB1 as determined by examining Hα-proton shifts relative to the random coil configuration. Delta (Δ)-values (random coil—experimental values) are presented where numerical values ± 0.1 ppm suggest a well-defined structure. Mutations sites are highlighted in red characters. The recognized H-proton shifts for residues Gly11-Gly12 and Ser22-Trp23 are indicated with gray filled bars. (C) NH-Hα fingerprint region of the TOCSY spectrum of [T20K]kB1 showing intraresidue connectivities. (D) Sequential dαN(i,i+1) connectivities in the NH-Hα fingerprint region of the NOESY spectrum for [T20K]kB1.