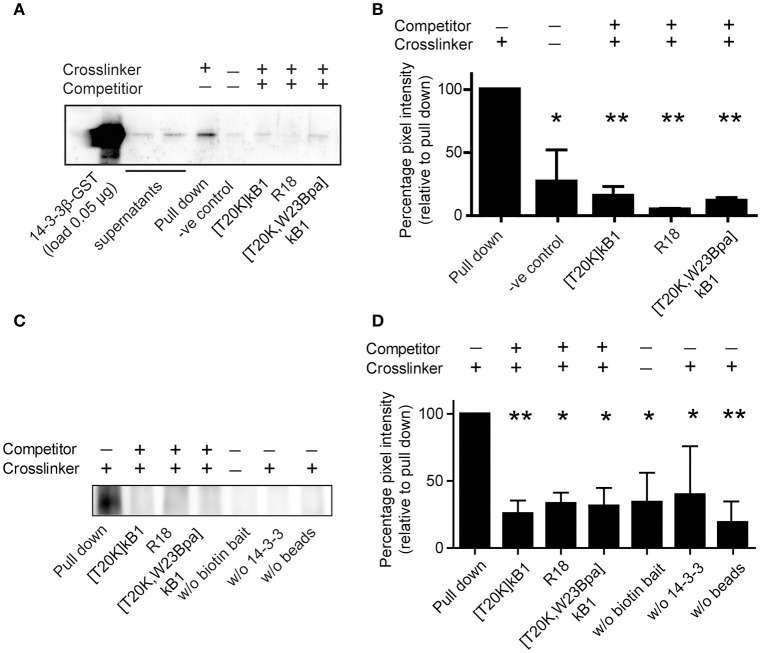
Figure 5.
Co-precipitation of purified GST-tagged isoform 14-3-3β with cyclotide probe [T20K,W23Bpa]kB1. (A) Protein/peptide interaction was confirmed by co-precipitation of representative recombinant GST-tagged 14-3-3β isoform with biotinylated crosslinker probe [T20K,W23Bpa]kB1. Validation of the interaction was obtained by competition experiments using non-biotinylated competitors [T20K]kB1, R18, and [T20K,W23Bpa]kB1, respectively. Beads and crosslinker probe [T20K,W23Bpa]kB1 only, were used as a control. After precipitation of biotinylated analytes with streptavidin beads, 14-3-3 positive samples were identified using an anti-GST primary antibody. A representative immunoblot is shown. (B) Pixel quantification of two independent immunoblots revealed significant reduction of the GST-tagged 14-3-3β isoform in the presence of non-biotinylated competitors. (C) In vitro co-precipitation was performed using the biotin tag for the precipitation to test for any binding of the probe to the GST tag of the 14-3-3β protein. As control the following conditions were used: (i) samples without biotinylated crosslinker, (ii) samples without 14-3-3β GST protein and (iii) samples without streptavidin beads. A representative immunoblot of three independent experiments is shown. (D) Pixel quantification of protein bands of the 55 kDa band (14-3-3β conjugated to a GST-tag and crosslinked to the biotinylated cyclotide probe [T20K,W23Bpa]kB1). Significance was analyzed by one-way ANOVA analysis with Bonferroni Post-Hoc test; *P < 0.05, **P < 0.01.