Abstract
Objective
The critical role of adipose tissue in energy and nutrient homeostasis is influenced by many external factors, including overnutrition, inflammation, and exogenous hormones. Prior studies have suggested that glucocorticoids (GCs) in particular are major drivers of physiological and pathophysiological changes in adipocytes. In order to determine whether these effects directly require the glucocorticoid receptor (GR) within adipocytes, we generated adipocyte-specific GR knockout (AGRKO) mice.
Methods
AGRKO and control mice were fed chow or high fat diet (HFD) for 14 weeks. Alternatively, AGRKO and control mice were injected with dexamethasone for two months. Glucose tolerance, insulin sensitivity, adiposity, lipolysis, thermogenesis, and insulin signaling were assessed.
Results
We find that obesity, insulin resistance, and dysglycemia associated with high fat feeding do not require an intact GR in the adipocyte. However, exogenous dexamethasone (Dex) promotes metabolic dysfunction in mice, and this effect is reduced in mice lacking GR in adipocytes. The ability of Dex to promote “whitening” of brown fat is also reduced in these animals. We also show that GR is required for β-adrenergic and cold stimulation-mediated lipolysis via expression of the key lipolytic enzyme ATGL.
Conclusions
Our data suggest that the GR plays a role in normal adipose physiology via effects on lipolysis and mediates at least some of the adverse effects of exogenous steroids on metabolic function. The data also indicate that intra-adipocyte GR plays less of a role than previously believed in the local and systemic pathology associated with overnutrition.
Keywords: Glucocorticoid receptor, Adipose tissue, Insulin resistance, Lipolysis, Dexamethasone
Highlights
-
•
Adipocyte glucocorticoid receptor plays a surprisingly minor role in obesity-induced insulin resistance.
-
•
Adipocyte glucocorticoid receptor partially mediates dexamethasone-induced insulin resistance.
-
•
Glucocorticoid receptor mediates dexamethasone-induced brown fat lipid accumulation.
-
•
Adipocyte glucocorticoid receptor is critical for lipolysis.
Abbreviations
- GCs
Glucocorticoids
- GR
Glucocorticoid receptor
- MR
Mineralocorticoid receptor
- AGRKO
Adipocyte-specific GR knock out
- Flox
GRflox/flox mice
- HFD
High fat diet
- Dex
Dexamethasone
- 11β-HSD1
11β-Hydroxysteroid dehydrogenase 1
- eWAT
Epididymal white adipose tissue
- iWAT
Inguinal white adipose tissue
- NEFA
Nonesterified fatty acids
- TG
Triglycerides
- TRAP
Translating ribosome affinity purification
- MRI
Magnetic resonance imaging
- Gas
Gastrocnemius
- Sol
Soleus
1. Introduction
Adipose tissue plays an important role in energy homeostasis, serving not only as the primary depot for caloric storage but also as an integrator of nutrient, hormonal, and immune signaling [1]. Under normal conditions, adipose tissue regulates processes as varied as appetite, thermogenesis, the fasting–feeding transition, blood pressure, and others. In overnutrition, adipose tissue becomes dysfunctional, developing progressive insulin resistance within the adipocyte compartment itself and ultimately contributing to systemic alterations in insulin sensitivity and lipid handling.
Among the hormonal factors that participate in the control of adipose behavior, glucocorticoids (GCs) are among the best studied. GCs (mainly cortisol in man and corticosterone in rodents) are produced by the adrenal cortex under the control of the hypothalamus and pituitary gland. GCs have been implicated in multiple aspects of adipose tissue biology, including fat cell differentiation [2], [3], [4], lipogenesis and lipolysis [5], and thermogenesis [6], [7], [8], [9], [10]. Exposure to high levels of endogenous or exogenous GCs causes profound adipose remodeling, including central obesity and peripheral wasting, as well as severe insulin resistance and hyperglycemia [11]. In mice, administration of GCs causes metabolic dysfunction that is highly reminiscent of that seen in human studies and transgenic overexpression of 11β-hydroxysteroid dehydrogenase 1 (11β-HSD1) in fat, which increases active GC levels and leads to increased adipose tissue mass, insulin resistance, and diabetes [12].
In our prior work, we have focused on the mechanisms by which cellular insulin resistance develops in adipocytes. We noted that insulin resistance is a common phenotype associated with both pro-inflammatory cytokines and anti-inflammatory GCs like dexamethasone (Dex), and we utilized genomic and epigenomic approaches to identify common pathogenic pathways [13], [14]. Interestingly, the glucocorticoid receptor (GR, encoded by Nr3c1) was implicated in these studies as a driver of insulin resistance in the presence of either Dex or TNF-α.
Here we explore three questions: 1) To what extent does GR within adipocytes participate in the development of insulin resistance in living animals, 2) do exogenous GCs cause metabolic dysfunction by acting through the GR in adipocytes, and 3) what other adipocyte functions are regulated by intrinsic GR? To address these questions, we deleted the GR selectively in adipocytes using the cre-loxP system in mice. We demonstrate that adipocyte GR participates in lipolysis but, surprisingly, does not contribute to altered glucose homeostasis or insulin resistance in the setting of diet-induced obesity. Administration of Dex, however, causes insulin resistance that depends upon the presence of GR in adipocytes.
2. Methods
2.1. Animal experiments
To generate AGRKO mice, we crossed Adiponectin-Cre mice (Jackson Laboratory, 010803) [15] with GR floxed mice (Jackson Laboratory, 021021). For high fat feeding studies, 6-week old littermate Flox (GRflox/flox) and AGRKO mice were fed 58% high fat diet (12331i, Research Diets) for 14 weeks. For studies using dexamethasone (Dex), 16-week old littermate Flox and AGRKO mice were administered saline or dexamethasone sodium phosphate (3 mg/kg body weight; Santa Cruz Biotechnology) intraperitoneally every other day for 2 months. Body weight was measured weekly. Mice were subjected to magnetic resonance imaging (MRI) (Echo Medical Systems) to examine body compensation. Tissues were harvested, frozen in liquid nitrogen, and stored at −80 °C until used. For cold exposure experiments, mice were placed at 4 °C for up to 6 h. Body temperature was measured using a rectal probe (Yellow Spring Instruments). All experiments were approved by the Beth Israel Deaconess Medical Center Institutional Animal Care and Use Committee.
2.2. Glucose and insulin tolerance test
For glucose tolerance tests, mice were fasted for 12 h and injected with glucose intraperitoneally (2 g/kg body weight for HFD studies; 1 g/kg body weight for Dex studies). Blood samples were collected at 0, 15, 30, 60, 90, and 120 min. For insulin tolerance tests, mice were fasted for 5 h and injected with insulin (1.5 U/kg body weight for HFD studies; 1 U/kg body weight for Dex studies). Blood samples were collected at 0, 15, 30, 60, 90, and 120 min. Glucose levels were measured with a handheld glucometer (One Touch Ultra Mini).
2.3. RNA isolation and quantitative PCR
Total RNA was extracted from tissues using TRIzol reagent (Invitrogen) following the manufacturer's instructions. Total RNA (500 ng) was converted into cDNA, and quantitative PCR (qPCR) was performed using SYBR Green qPCR Master Mix (Applied Biosystems) using a 7900HT Fast Real Time PCR system. The relative abundance of mRNA was standardized with 36B4 mRNA as the invariant control. Primer sequences are provided in Supplemental Table 1.
2.4. Protein extraction and western blotting
Protein was extracted from tissues in RIPA buffer (Boston BioProducts) supplemented with complete protease inhibitor cocktail (Roche). Fat pads were fractionated into adipocytes and non-adipocytes as described [16]. Lysates were separated by 4–15% gradient SDS-PAGE and transferred to PVDF membrane (Millipore). The following antibodies were used: Akt (#9272), p-Akt (S473) (#9271), p70S6K (#2708), p-p70S6K (#9205), β-actin (#4970), and α/β-tubulin (#2148), all from Cell Signaling Technology. GR (M-20) was from Santa Cruz. UCP-1 (ab10983), total OXPHOS (ab110143), and Tomm20 (ab56783) were from Abcam. All blots were quantified using ImageJ software (National Institutes of Health, Bethesda, MD).
2.5. Histology
Collected fat tissues were fixed in 4% formaldehyde for 48 h at 4 °C and washed with PBS. Paraffin embedded tissues were stained with H&E, and images were acquired using a Zeiss-Axio Imager A1 microscope. Adipocyte size was quantified using ImageJ software (NIH).
2.6. Plasma parameters
Blood samples were collected from fasted mice in EDTA-coated blood collection tubes and plasma insulin levels were measured by ELISA kit according to the manufacturer's instructions (Crystal Chem). Plasma levels of triglycerides and nonesterified fatty acids (NEFA) were determined using kits from Thermo Scientific and Wako Diagnostics, respectively.
2.7. Lipolysis assay
For in vivo studies, mice were fasted for 4 h and injected with isoproterenol (10 mg/kg body weight). Blood was collected from the tail vein before and 20 min after injection. Glycerol was determined using a kit from Sigma–Aldrich, and NEFA content was measured as above. For ex vivo studies, epididymal fat pads were surgically removed from male mice and washed with ice-cold PBS. Excised adipose tissue pads (10–20 mg) were incubated in the presence or absence of 1 mM isoproterenol with DMEM for 0–2 h at 37 °C with gentle shaking.
2.8. Insulin signaling
Mice were fasted overnight, followed by IP insulin injection (10 U/kg body weight). Ten minutes later, tissues were collected and stored at −80 °C until use. Tissue samples were homogenized in RIPA buffer containing protease inhibitor (Roche) and phosphatase inhibitor (Sigma–Aldrich) and subjected to western blotting.
2.9. Statistical analysis
All data are presented as mean ± SEM. Unpaired two-tailed student's t-test and two-way ANOVA were used. p Values < 0.05 were considered statistically significant.
3. Results
3.1. Adipocyte GR is not required for the development of insulin resistance after high-fat diet feeding
To determine whether GR in mature adipocytes mediates insulin resistance in vivo, we generated adipocyte-specific GR knock out (AGRKO) mice by crossing adiponectin-Cre mice [15] with GRflox/flox (hereafter referred to as Flox) mice. AGRKO mice displayed reduced GR protein levels in BAT and epididymal WAT (eWAT), while displaying normal expression in non-adipose tissues such as liver, spleen, and muscle (Supplementary Figure 1A). The residual GR protein in AGRKO adipose tissue is likely from non-adipocytes, which make up at least half of the cells in a fat pad [17]. To prove this, we isolated the mature adipocyte fraction from inguinal white adipose tissue (iWAT) and eWAT and noted near complete absence of GR protein in isolated adipocytes of AGRKO mice (Supplementary Figure 1B). Deletion of GR in adipocytes had no effect on body weight and body composition on chow diet (Supplementary Figure 1C and D). AGRKO mice did not have different fasting plasma insulin levels (Supplementary Figure 1E). In addition, they did not show differences in glucose tolerance or insulin sensitivity (Supplementary Figure 1F and G).
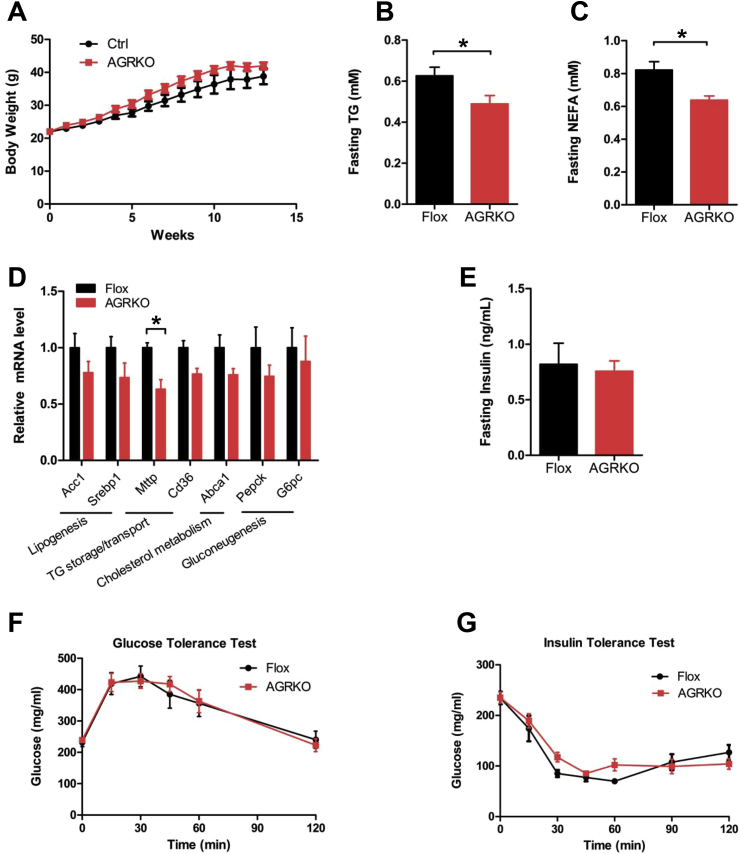
To test the role of adipocyte GR in the development of insulin resistance associated with diet-induced obesity, we fed Flox and AGRKO mice a high-fat diet (HFD) for 14 weeks. Both genotypes gained weight on the diet, and although AGRKO mice displayed a trend towards more weight, the difference was not significant (Figure 1A). Histological analysis of eWAT, iWAT, and BAT also showed no obvious differences between the genotypes (Supplementary Figure 2A). By contrast, we found that AGRKO mice had significantly decreased levels of plasma triglycerides (TG) and non-esterified fatty acids (NEFA) compared to control mice (Figure 1B and C). Gene expression analysis of eWAT indicated that most of the genes assessed were not differentially expressed in AGRKO mice, with the exception of a modest elevation in Gpat4 (Supplementary Figure 2B). Liver of AGRKO mice showed a trend toward a decrease of genes involved in lipid metabolism and gluconeogenesis with significant decrease of Mttp (microsomal triglyceride transfer protein (Figure 1D)), suggesting that TG and lipid homeostasis is slightly changed in AGRKO mice on HFD. Next, to study glucose homeostasis of AGRKO mice in the setting of diet-induced obesity, we measured fasting plasma insulin levels, which were not different between the two genotypes (Figure 1E). Similarly, glucose and insulin tolerance tests were not distinguishable between AGRKO and Flox control mice (Figure 1F and G).
Figure 1.
Adipocyte-specific deletion of GR causes minimal effects on glucose homeostasis in diet-induced obesity. (A) Body weight of WT and AGRKO mice during HFD feeding (n = 5 per group). Plasma TG level (B) and NEFA level (C) in Flox and AGRKO mice after 14 weeks of HFD feeding. (D) Gene expression analysis of liver in Flox and AGRKO mice after HFD feeding. (E) Fasting plasma insulin levels in Flox and AGRKO mice. (F) Glucose tolerance test in Flox and AGRKO mice after 12 weeks on HFD. (G) Insulin tolerance test in Flox and AGRKO mice after 13 weeks on HFD. Data are shown as mean ± SEM. *p < 0.05.
We considered whether compensation by the mineralocorticoid receptor (MR, encoded by Nr3c2) could account for the lack of strong effect of GR ablation. Prior studies on MR abundance have used whole tissue, so we examined both whole tissue and intra-adipocyte Nr3c1 and Nr3c2 levels, using RNA-seq data obtained by Translating Ribosome Affinity Purification (TRAP), reported previously [17]. These data indicate that the GR is expressed at approximately 20-fold greater levels than MR in adipocytes (Supplementary Figure 3A). Furthermore, adipose Nr3c2 levels did not rise in compensation in high fat fed AGRKO mice (Supplementary Figure 3B), suggesting that MR is unlikely to compensate for the loss of GR.
3.2. Dexamethasone administration increases adiposity independently of GR in adipocytes
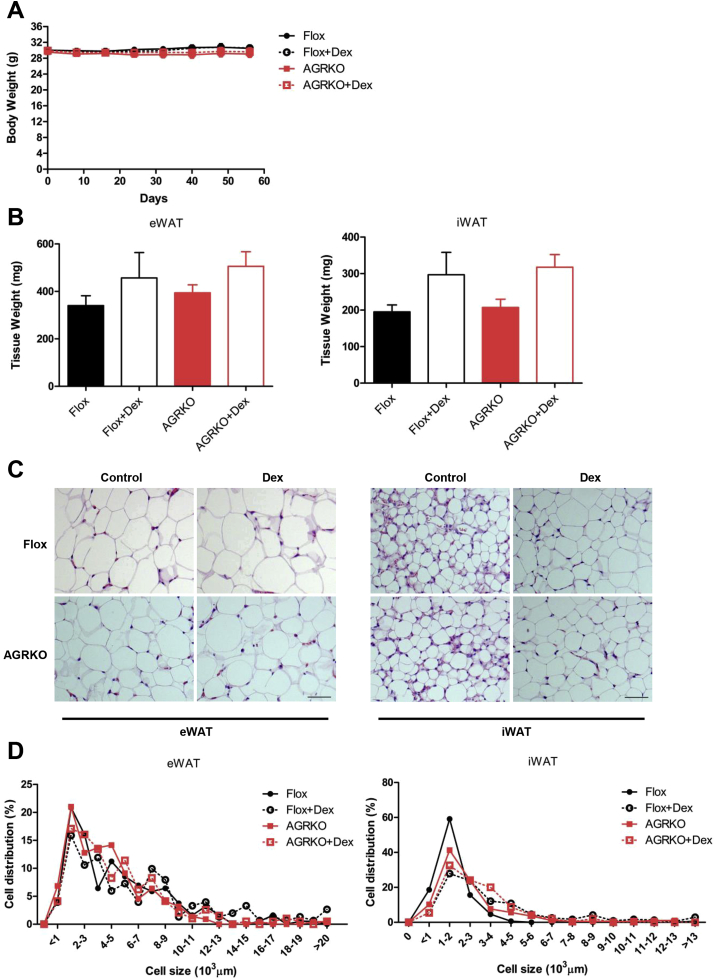
As we found a minor role of adipocyte GR in the development or complications of diet-induced obesity, we next examined the role of adipocyte GR in metabolic disorders induced by exogenous glucocorticoids. We chose Dex as the agent to administer, because it does not cross-react with the mineralocorticoid receptor; this allows us to more purely test the effects of GR. We gave a low dose of Dex or saline vehicle to the Flox control and AGRKO mice, starting at the age of 4 months, every two days for 8 weeks. This Dex regimen did not affect body weight in either group (Figure 2A), but Dex-treated mice displayed a trend toward increased adipose tissue (eWAT and iWAT) weight compared to saline-treated controls in both genotypes (Figure 2B). Histological analysis showed that Dex treatment increased adipocyte size in eWAT and iWAT, but had a more obvious effect on iWAT in both Flox and AGRKO groups (Figure 2C,D). These results suggest that Dex treatment increases adipocyte hypertrophy, especially in iWAT, but this effect is independent of the action of GR in adipocytes.
Figure 2.
Dexamethasone administration increases adiposity independently of GR in adipocytes. Flox and AGRKO mice were injected with saline or dexamethasone (Dex, 3 mg/kg body weight) every other day for two months (n = 8–9 per group). (A) Body weight, (B) adipose tissue weights, and (C) histology (H&E staining) of eWAT and iWAT of Flox and AGRKO mice injected with saline or Dex. (D) The distribution of adipocyte size calculated using ImageJ software. Data are shown as mean ± SEM. Scale bars indicate 100 μm.
3.3. AGRKO mice are protected from Dex-induced insulin resistance
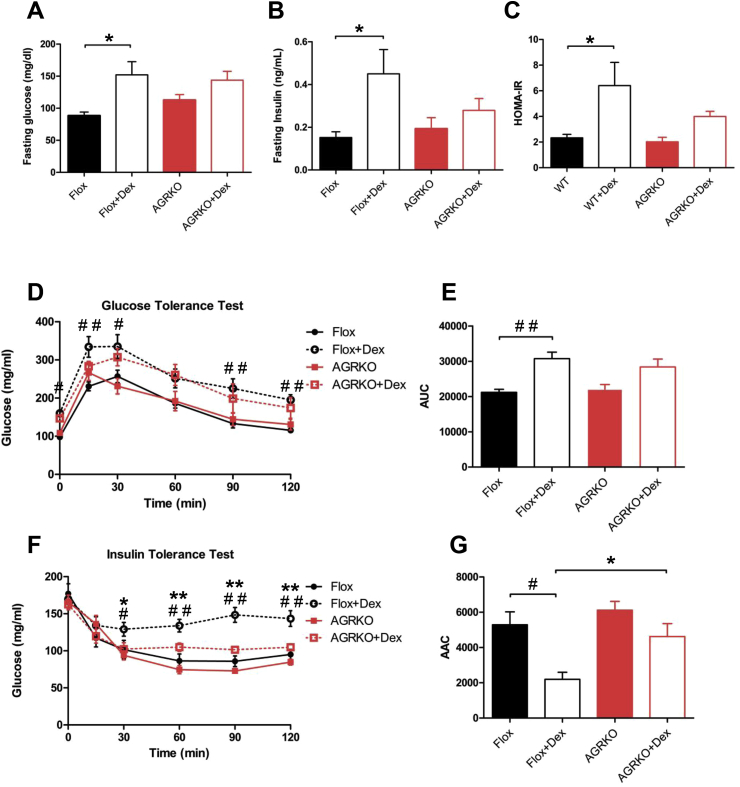
Glucocorticoids generally, and Dex in particular, are well-known to cause dysglycemia and insulin resistance in rodents and humans [11], [12], although it is not known if the adipocyte is a relevant site of action for this effect. To assess whether the adipocyte GR participates in the altered glucose homeostasis associated with pharmacological treatment with glucocorticoid, we analyzed several metabolic parameters in Flox and AGRKO mice. In Flox mice, Dex treatment resulted in elevated fasting glucose, fasting insulin levels, and increased HOMA-IR, a measure of insulin resistance (Figure 3A–C). Dex caused a similar but slightly smaller than normal increase in fasting glucose levels in AGRKO mice (Figure 3A). However, fasting insulin levels and HOMA-IR were not as Dex-inducible in the absence of GR (Figure 3B and C). Similarly, glucose tolerance testing revealed that Flox mice treated with Dex displayed worse glucose intolerance relative to mice treated with saline (Figure 3D and E). AGRKO mice, on the other hand, showed less pronounced glucose intolerance after Dex treatment (Figure 3D and E). This effect was even stronger in an insulin tolerance test, in which Dex caused a significant degree of insulin resistance in Flox mice but not in AGRKO mice (Figure 3F and G). Taken together, these results indicate that GR in adipocytes plays an important role in glucocorticoid-mediated insulin resistance.
Figure 3.
AGRKO mice are protected from Dex-induced insulin resistance. (A) Fasting blood glucose, (B) plasma insulin, and (C) HOMA-IR of Flox and AGRKO mice injected with saline or Dex. (D) Glucose tolerance test (GTT) (1 mg/kg body weight) performed after six weeks of Dex injection; (E) Glucose area under the curve (AUC) of the GTT; (F) Insulin tolerance test (ITT) (1U/kg body weight) performed after seven weeks of Dex injection (G) Insulin area above the curve (AAC) of the ITT. Data are shown as mean ± SEM. *, #p < 0.05, **, ##p < 0.01.
3.4. Loss of GR in adipocytes protects from Dex-induced muscle insulin resistance
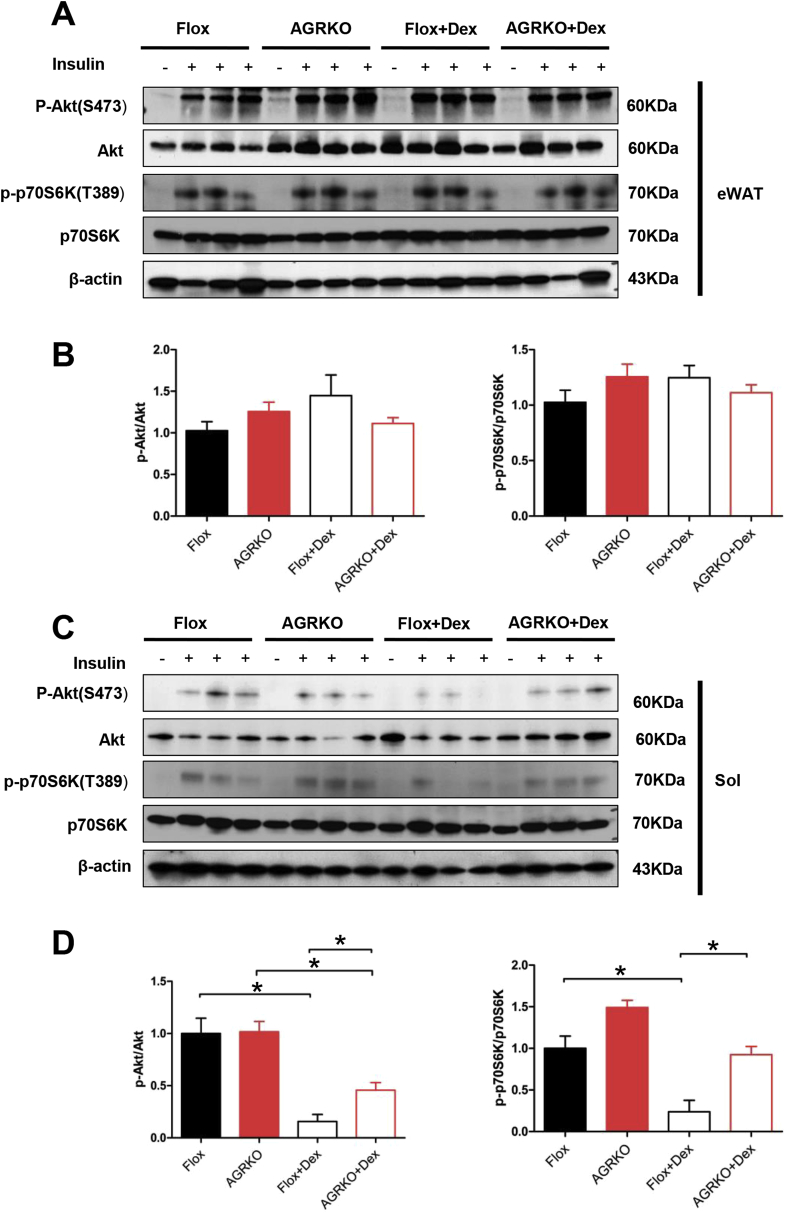
To determine the contribution of different metabolic organs to systemic insulin sensitivity after Dex administration, we tested the insulin signaling pathway in eWAT, liver, and muscle (gastrocnemius and soleus). Consistent with our prior results in cultured adipocytes [3], Dex did not reduce Akt and p70S6K phosphorylation in eWAT (Figure 4A and B). A similar result was seen in liver (Supplemental Figure 4C and D). Intriguingly, however, both soleus and gastrocnemius muscles showed reduced Akt or p70S6K phosphorylation in the presence of Dex, and this effect was significantly (though not completely) abrogated in AGRKO mice (Figure 4C, D and Supplementary Figure 4A, B). These data indicate that muscle insulin resistance induced by Dex-treatment was partially rescued by the loss of GR in adipocytes. Since Dex treatment is known to cause muscle atrophy [18], we tested whether muscle insulin sensitivity is linked with muscle atrophy. In Flox mice, Dex treatment induced the expression of several muscle atrophy marker genes, such as Fbxo32/Atrogin-1, Foxo3, Trim64/Murf1 and Pik3r1/p85. Similar Dex-induced gene expression changes were observed in AGRKO mice (Supplementary Figure 4E). These results indicate that the improved muscle insulin sensitivity of AGRKO mice in the setting of Dex administration is likely to be independent of changes in muscle atrophy.
Figure 4.
Loss of GR in adipocyte protects Dex-induced muscle insulin resistance. (A) Western blot analysis of insulin-stimulated Akt and p70S6K phosphorylation in eWAT of Flox and AGRKO mice injected with saline or Dex. Quantitation of western blot in eWAT is shown in (B). (C) Western blot analysis of insulin-stimulated Akt and p70S6K phosphorylation in soleus. Quantitation of western blot in soleus is shown in (D). Data are shown as mean ± SEM. *p < 0.05.
3.5. Dex causes lipid accumulation in brown fat in a GR-dependent manner
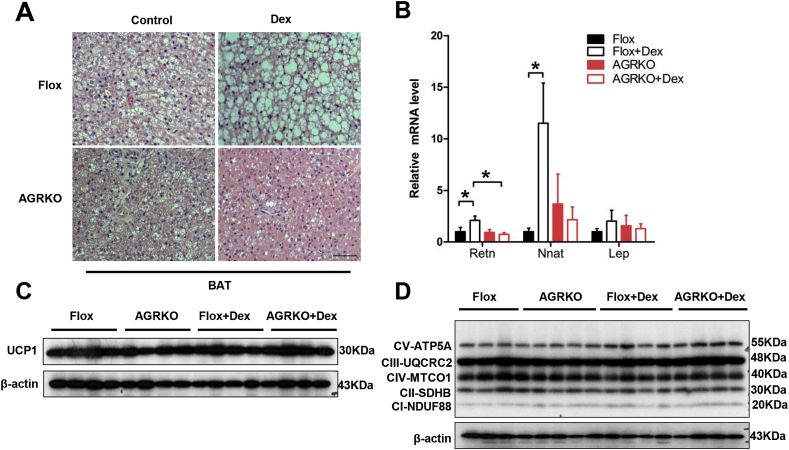
Dex changed the histological appearance of BAT, causing lipid accumulation and an apparent whitening of the tissue (Figure 5A). This was associated with the induction of several genes of white adipose tissue, such as Retn and Nnat (but not Lep) (Figure 5B). Importantly, ablation of GR in adipocytes blocked these “whitening” actions of Dex (Figure 5B). Glucocorticoids have been shown to suppress Ucp1 expression in cultured rodent adipocytes [6], [10]. Interestingly, our Dex regimen did not change the level of UCP-1 protein (Figure 5B). The absence of GR also did not significantly alter mitochondrial protein levels, in either the presence or absence of Dex (Figure 5C). Taken together, these data suggest that the increased lipid accumulation in BAT after Dex may not be due purely to reduced thermogenesis but may also involve a lipogenic or pro-whitening effect that does not require suppression of the classical BAT signature.
Figure 5.
Dex does not cause lipid accumulation in BAT of AGRKO mice. (A) Histology (H&E staining) of brown fat in Flox and AGRKO mice injected with saline or Dex. Scale bars indicate 100 μm (B) qRT-PCR analysis of white adipocyte marker genes in brown fat. Western blot analysis of UCP1 (C) and mitochondrial complex proteins (D) in Flox and AGRKO mice. Data are shown as mean ± SEM. *p < 0.05.
3.6. Adipocyte GR is required for lipolysis
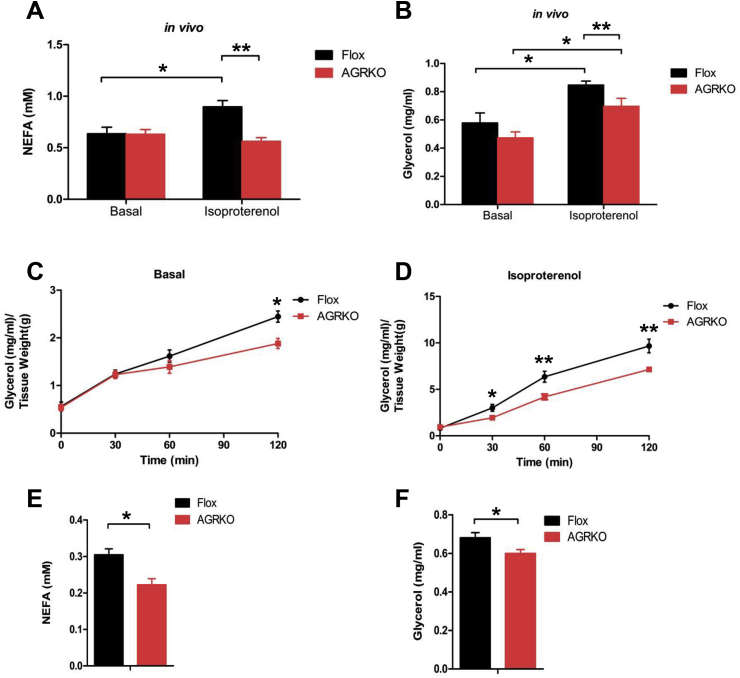
Reduced NEFA in AGRKO mice (Figure 1C) suggested that adipocyte GR may play a role in lipolysis. We therefore measured plasma glycerol and NEFA under basal (4 h fasted) and isoproterenol-stimulated conditions. Basal levels of plasma NEFA and glycerol in vivo were not different between the genotypes, but isoproterenol-stimulated lipolysis was significantly reduced in AGRKO (Figure 6A and B). Similar lipolysis defects were observed with adipose explants; both basal (120 min) and isoproterenol-stimulated glycerol release was reduced in AGRKO mice (Figure 6C and D). When exposed to 4 h cold challenge (a physiological lipolytic stimulation), AGRKO mice displayed significant lower glycerol and NEFA levels compared to Flox mice (Figure 6E and F). Taken all together, these data indicate that GR is required for normal adipocyte lipolysis.
Figure 6.
Adipocyte GR is required for lipolysis in vivo. Lipolysis in Flox and AGRKO mice on chow diet. NEFA (A) and glycerol (B) in plasma were measured in the absence and presence of isoproterenol. (C and D) Rate of lipolysis in adipose explants isolated from epididymal fat of male Flox and AGRKO mice. Glycerol was measured in the absence and presence of 1 μM of isoproterenol. NEFA (E) and glycerol (F) in plasma were measured after 4 h cold exposure (4 °C). Data are shown as mean ± SEM. *p < 0.05, **p < 0.01.
To dissect out the mechanism by which GR regulates lipolysis, we analyzed our published H3K27ac ChIP-seq data with 3T3-L1 adipocytes treated with Dex to determine whether the expression of key lipolytic genes, including Plin1, Pnpla2 (encoding adipose triglyceride lipase; ATGL), Lipe (encoding hormone-sensitive lipase; HSL), and Mgll, is changed in response to Dex treatment in adipocytes. We found that only Pnpla2 displayed increased H3K27ac peak activity in response to Dex (Supplementary Figure 5A). We then measured the expression of these lipolysis genes in eWAT after Dex treatment and found Pnpla2 was significantly increased by Dex while Mgll was unchanged, and Plin1 and Lipe were reduced, suggesting that Pnpla2 is a key target gene of the GR in adipocytes (Supplementary Figure 5B). Consistently, we observed that Pnpla2 expression was significantly decreased in AGRKO mice compared to Flox mice, while other lipolysis genes were unchanged (Supplementary Figure 5C).
4. Discussion
The GR is a transcription factor expressed in nearly all vertebrate cell types. In response to circulating glucocorticoids, the GR coordinates an enormous range of transcriptional programs with effects on cellular differentiation, the stress response, inflammation, and many other biological processes [19]. In adipose tissue, GCs have been implicated in adipogenesis, as well as lipolysis, lipogenesis, and insulin resistance [2], [3], [5], [11]. GCs also repress Ucp1 expression and reduce thermogenesis in vivo and in cultured rodent brown adipocytes, although they may have different effects in humans [6], [7], [8], [9], [10]. Finally, GCs are also associated with significant remodeling of adipose tissue, with central obesity and supraclavicular fat deposition noted in patients with both pharmacological and endogenous GC excess. Adipose-specific overexpression of 11-βHSD1, which increases local concentrations of active GC, causes visceral obesity and insulin resistance, while overexpression of 11-βHSD2, which inactivates local GCs, has the opposite effects [12], [20]. Importantly, our prior data using cultured adipocytes demonstrated a role for the GR in mediating cellular insulin resistance in response to both Dex and TNF-α [14].
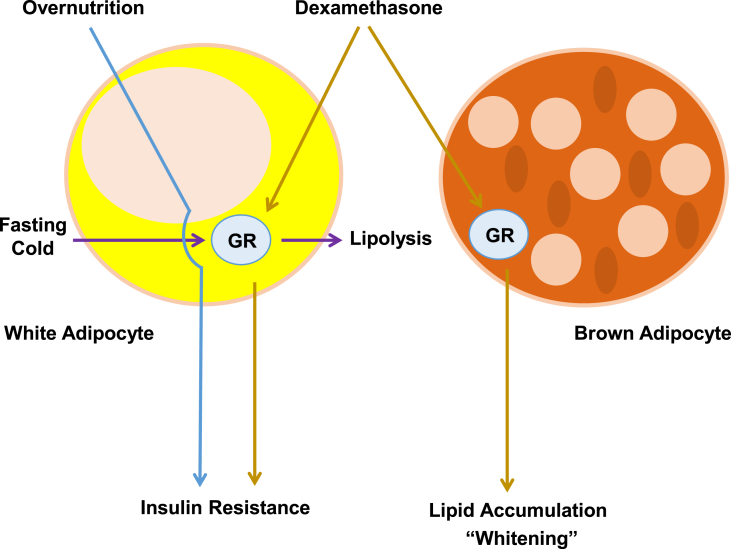
We sought to ascertain which actions of GCs are mediated directly through adipose tissue in vivo, using Cre-lox technology to selectively delete the GR in white and brown adipocytes. Despite the vast amount of suggestive data mentioned above, our results surprisingly indicate that adipocyte GR is not required for the glucose intolerance and insulin resistance associated with diet-induced obesity. We also asked whether exogenous GCs work through the adipocyte GR to affect metabolism. Dex did not cause weight gain in our model, but there was a trend towards increased adiposity that was not dependent on the presence of GR in white adipocytes. In BAT, however, Dex caused a large increase in lipid accumulation and the expression of several genes associated with “whitening”, and this effect absolutely required GR to be present in adipocytes (Figure 7). Interestingly, our Dex regimen did not affect UCP1 protein expression, although others have reported a reduction [6]. Most interestingly, Dex-induced insulin resistance was ameliorated in AGRKO mice (though not Dex-induced glucose intolerance), and this effect was associated with improved insulin signaling in muscle, but not WAT.
Figure 7.
Schematic summarizing the function of GR in adipocytes. Adipocyte GR is not required for overnutrition-induced insulin resistance. However, adipocyte GR mediates dexamethasone-induced insulin resistance and brown adipocyte lipid accumulation and the expression of genes associated with “whitening”. Adipocyte GR also mediates fasting and cold-stimulated lipolysis.
This is not to say that the adipocyte GR plays no role in energy metabolism, as AGRKO mice exhibit reduced catecholamine-stimulated lipolysis in vivo and ex vivo (Figure 7). This effect seems likely to be mediated through GR-induced expression of Pnpla2, as this gene was concordantly affected by Dex and by loss of GR.
It should be pointed out that there have been other recent studies of AGRKO mice, and as is typical of metabolic phenotyping studies there are some striking similarities as well as some important differences relative to our findings. Consistent with our work, Desarzens et al. [21] and Bose et al. [22] reported no change in body weight, insulin, or glucose tolerance on a high fat diet. Mueller et al. [23], on the other hand, reported that AGRKO mice were somewhat leaner and more insulin sensitive than control littermates in the setting of both aging and 20 weeks of high fat feeding. As in our study, this last group also showed that AGRKO mice have a defect in lipolysis. Only one other study tested the effect of Dex administration in AGRKO mice; Bose et al. saw a trend toward improvement in insulin action after Dex that ultimately failed to meet significance. Liver-specific GRKO mice, however, were somewhat protected from the metabolic consequences of Dex in that study.
Although all of these findings are not fully reconcilable, taken together the majority of the data suggests that the GR in adipocytes may play a less important role than previously thought in insulin resistance caused by overnutrition (Figure 7). In this light, it is worth pointing out that there has also been a recent re-evaluation of what had been considered to be a critical role for the GR in adipocyte differentiation. In these studies, cells without GR were fully able to differentiate in vitro, although the process was a bit delayed, while fat formation in vivo was unaffected by the absence of GR [24].
Another recent study showed that GCs require the presence of the pioneer factor Foxa2 in adipocytes in order to mediate some, but not all, gene expression events [25]. Genes of lipid handling, such as Lipe and Lpin1 (encoding a key enzyme in triglyceride synthesis) were notably dependent upon Foxa2. Furthermore, in this study Dex caused obesity, but only when Foxa2 was present. In our work, we saw a reduction in Lipe and Lpin1 levels in AGRKO mice after high fat feeding that did not quite reach significance (Supplementary Figure 2B). In addition, our Dex regimen was able to promote insulin resistance in the absence of weight gain, demonstrating that enhanced adiposity is not required for GCs to alter glycemia.
The effect of GCs on brown adipose tissue is complex, and may be different between rodents and humans [6], [7], [9], [10]. As with white fat, GCs have long been considered to be critical for brown adipogenesis, although recent data refute this notion. Mice lacking the GR in the Myf5+ lineage that gives rise to BAT have normal BAT amounts and function [24]. Treatment of murine brown adipocytes ex vivo or in vitro with GCs typically reduces Ucp1 expression, an effect that is especially pronounced when basal Ucp1 levels are stimulated by the addition of a β-adrenergic agonist [26]. However, UCP1 and mitochondrial electron transport chain protein levels in our data are not altered by either the absence of the GR or by the addition of Dex. Instead, we did see an increase in lipid accumulation in BAT from Dex-treated mice, an effect not noted when the GR was ablated. This is consistent with data from the Harris group, who reported lipogenic gene induction in BAT after Dex administration (e.g. Dgat2, Gpat4, Lpin1), which was blocked in their AGRKO mice [22]. Taken together, our data suggest that exogenous GCs may affect lipid handling in brown adipocytes directly through local GR, but the GR is unlikely to exert large effects on the thermogenic function of BAT.
One possible interpretation of the finding that the adipocyte GR is dispensable for the development of diet-induced obesity and insulin resistance is that the MR may play a more important role in these processes. Endogenous GCs, such as cortisol in humans and corticosterone in rodents, can act as agonists of the mineralocorticoid receptor and, in fact, have ten-fold higher affinity for the MR than for the GR [27]. Furthermore, MR expression has been reported to be elevated in the adipose tissue of obese rodents and humans, and overexpression of MR in fat causes obesity and insulin resistance [28], [29]. Finally, the MR antagonist eplerenone reverses the metabolic dysfunction associated with obesity in mice [30]. It is worth noting, however, that prior studies showing elevated MR levels in adipose tissue have been done with whole tissue samples, which contain a mixture of cell types. We have obtained sequence-based (i.e. not prone to hybridization artifact) gene expression profiles from a pure population of translating ribosomes, which reveal very low Nr3c2 mRNA levels in adipocytes. Furthermore, MR levels do not increase in AGRKO mice. A definitive experiment would involve studying a combined adipocyte-specific GR and MR double knockout mouse, but this has never been done. Certainly these issues motivated, in part, our choice of dexamethasone as an exogenous GC, as Dex is devoid of MR agonist activity.
One interesting result from our study was that AGRKO mice were protected from Dex-mediated reduction of insulin signaling in muscle but not in fat. Although Dex can promote atrophy in muscle via intra-myocyte GR [31], atrophy-associated genes in muscle were not differentially expressed in AGRKO mice after Dex. It is possible that circulating factors secreted from adipose tissues affect muscle insulin action, such as resistin. Resistin mRNA levels rose in BAT in the presence of Dex, and this did not occur in AGRKO mice.
Taken as a whole, our data do not support a role for the adipocyte GR in the insulin resistance associated with diet-induced obesity. This is in accordance with most, but not all, of the recent data from other groups. It is not unusual for metabolic phenotyping data to be discordant across different laboratories, even when background strain and other mouse-intrinsic variables are held rigorously constant [32]. At a minimum, the emerging picture strongly suggests that a demonstrable role for adipocyte GR in the metabolic dysfunction of high-fat feeding is not robust or highly reproducible.
Author contributions
YS, HCR, and EDR conceived of the experimental plan. YS, HCR, and MK performed experiments and analyzed the data. YS, HCR, and EDR wrote the manuscript.
Acknowledgements
The authors gratefully acknowledge members of the Rosen lab for their helpful advice and discussion. This work was supported by a China Scholarship Council Scholarship to YS, a Charles Hood Trust Fellowship to HCR, and NIH R01 DK102173, R01 DK085171, and DK102170 to EDR.
Footnotes
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.molmet.2017.06.013.
Conflicts of interest
None declared.
Appendix A. Supplementary data
The following are the supplementary data related to this article:
References
- 1.Rosen E.D., Spiegelman B.M. What we talk about when we talk about fat. Cell. 2014;156(1–2):20–44. doi: 10.1016/j.cell.2013.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hauner H., Schmid P., Pfeiffer E.F. Glucocorticoids and insulin promote the differentiation of human adipocyte precursor cells into fat cells. Journal of Clinical Endocrinology & Metabolism. 1987;64(4):832–835. doi: 10.1210/jcem-64-4-832. [DOI] [PubMed] [Google Scholar]
- 3.Pantoja C., Huff J.T., Yamamoto K.R. Glucocorticoid signaling defines a novel commitment state during adipogenesis in vitro. Molecular Biology of the Cell. 2008;19(10):4032–4041. doi: 10.1091/mbc.E08-04-0420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rosen E.D., MacDougald O.A. Adipocyte differentiation from the inside out. Nature Reviews Molecular Cell Biology. 2006;7(12):885–896. doi: 10.1038/nrm2066. [DOI] [PubMed] [Google Scholar]
- 5.Peckett A.J., Wright D.C., Riddell M.C. The effects of glucocorticoids on adipose tissue lipid metabolism. Metabolism. 2011;60(11):1500–1510. doi: 10.1016/j.metabol.2011.06.012. [DOI] [PubMed] [Google Scholar]
- 6.Ramage L.E., Akyol M., Fletcher A.M., Forsythe J., Nixon M., Carter R.N. Glucocorticoids acutely increase brown adipose tissue activity in humans, revealing species-specific differences in UCP-1 regulation. Cell Metabolism. 2016;24(1):130–141. doi: 10.1016/j.cmet.2016.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kong X., Yu J., Bi J., Qi H., Di W., Wu L. Glucocorticoids transcriptionally regulate miR-27b expression promoting body fat accumulation via suppressing the browning of white adipose tissue. Diabetes. 2015;64(2):393–404. doi: 10.2337/db14-0395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hardwick A.J., Linton E.A., Rothwell N.J. Thermogenic effects of the antiglucocorticoid RU-486 in the rat: involvement of corticotropin-releasing factor and sympathetic activation of brown adipose tissue. Endocrinology. 1989;124(4):1684–1688. doi: 10.1210/endo-124-4-1684. [DOI] [PubMed] [Google Scholar]
- 9.Strack A.M., Bradbury M.J., Dallman M.F. Corticosterone decreases nonshivering thermogenesis and increases lipid storage in brown adipose tissue. American Journal of Physiology. 1995;268(1 Pt 2):R183–R191. doi: 10.1152/ajpregu.1995.268.1.R183. [DOI] [PubMed] [Google Scholar]
- 10.van den Beukel J.C., Grefhorst A., Quarta C., Steenbergen J., Mastroberardino P.G., Lombes M. Direct activating effects of adrenocorticotropic hormone (ACTH) on brown adipose tissue are attenuated by corticosterone. The FASEB Journal. 2014;28(11):4857–4867. doi: 10.1096/fj.14-254839. [DOI] [PubMed] [Google Scholar]
- 11.Ferrau F., Korbonits M. Metabolic comorbidities in Cushing's syndrome. European Journal of Endocrinology. 2015;173(4):M133–M157. doi: 10.1530/EJE-15-0354. [DOI] [PubMed] [Google Scholar]
- 12.Masuzaki H., Paterson J., Shinyama H., Morton N.M., Mullins J.J., Seckl J.R. A transgenic model of visceral obesity and the metabolic syndrome. Science. 2001;294(5549):2166–2170. doi: 10.1126/science.1066285. [DOI] [PubMed] [Google Scholar]
- 13.Houstis N., Rosen E.D., Lander E.S. Reactive oxygen species have a causal role in multiple forms of insulin resistance. Nature. 2006;440(7086):944–948. doi: 10.1038/nature04634. [DOI] [PubMed] [Google Scholar]
- 14.Kang S., Tsai L.T., Zhou Y., Evertts A., Xu S., Griffin M.J. Identification of nuclear hormone receptor pathways causing insulin resistance by transcriptional and epigenomic analysis. Nature Cell Biology. 2015;17(1):44–56. doi: 10.1038/ncb3080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eguchi J., Wang X., Yu S., Kershaw E.E., Chiu P.C., Dushay J. Transcriptional control of adipose lipid handling by IRF4. Cell Metabolism. 2011;13(3):249–259. doi: 10.1016/j.cmet.2011.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eguchi J., Yan Q.W., Schones D.E., Kamal M., Hsu C.H., Zhang M.Q. Interferon regulatory factors are transcriptional regulators of adipogenesis. Cell Metabolism. 2008;7(1):86–94. doi: 10.1016/j.cmet.2007.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roh H.C., Tsai L.T., Lyubetskaya A., Tenen D., Kumari M., Rosen E.D. Simultaneous transcriptional and epigenomic profiling from specific cell types within heterogeneous tissues in vivo. Cell Reports. 2017;18(4):1048–1061. doi: 10.1016/j.celrep.2016.12.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bodine S.C., Furlow J.D. Glucocorticoids and skeletal muscle. Advances in Experimental Medicine & Biology. 2015;872:145–176. doi: 10.1007/978-1-4939-2895-8_7. [DOI] [PubMed] [Google Scholar]
- 19.Weikum E.R., Knuesel M.T., Ortlund E.A., Yamamoto K.R. Glucocorticoid receptor control of transcription: precision and plasticity via allostery. Nature Reviews Molecular Cell Biology. 2017;18(3):159–174. doi: 10.1038/nrm.2016.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kershaw E.E., Morton N.M., Dhillon H., Ramage L., Seckl J.R., Flier J.S. Adipocyte-specific glucocorticoid inactivation protects against diet-induced obesity. Diabetes. 2005;54(4):1023–1031. doi: 10.2337/diabetes.54.4.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Desarzens S., Faresse N. Adipocyte glucocorticoid receptor has a minor contribution in adipose tissue growth. Journal of Endocrinology. 2016;230(1):1–11. doi: 10.1530/JOE-16-0121. [DOI] [PubMed] [Google Scholar]
- 22.Bose S.K., Hutson I., Harris C.A. Hepatic glucocorticoid receptor plays a greater role than adipose GR in metabolic syndrome despite renal compensation. Endocrinology. 2016;157(12):4943–4960. doi: 10.1210/en.2016-1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mueller K.M., Hartmann K., Kaltenecker D., Vettorazzi S., Bauer M., Mauser L. Adipocyte glucocorticoid receptor deficiency attenuates aging- and HFD-induced obesity and impairs the feeding-fasting transition. Diabetes. 2017;66(2):272–286. doi: 10.2337/db16-0381. [DOI] [PubMed] [Google Scholar]
- 24.Park Y.K., Ge K. Glucocorticoid receptor accelerates, but is dispensable for, adipogenesis. Molecular and Cellular Biology. 2017;(2):37. doi: 10.1128/MCB.00260-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ma X., Xu L., Mueller E. Forkhead box A3 mediates glucocorticoid receptor function in adipose tissue. Proceedings of the National Academy of Sciences of the United States of America. 2016;113(12):3377–3382. doi: 10.1073/pnas.1601281113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Soumano K., Desbiens S., Rabelo R., Bakopanos E., Camirand A., Silva J.E. Glucocorticoids inhibit the transcriptional response of the uncoupling protein-1 gene to adrenergic stimulation in a brown adipose cell line. Molecular and Cellular Endocrinology. 2000;165(1–2):7–15. doi: 10.1016/s0303-7207(00)00276-8. [DOI] [PubMed] [Google Scholar]
- 27.Arriza J.L., Weinberger C., Cerelli G., Glaser T.M., Handelin B.L., Housman D.E. Cloning of human mineralocorticoid receptor complementary DNA: structural and functional kinship with the glucocorticoid receptor. Science. 1987;237(4812):268–275. doi: 10.1126/science.3037703. [DOI] [PubMed] [Google Scholar]
- 28.Nguyen Dinh Cat A., Antunes T.T., Callera G.E., Sanchez A., Tsiropoulou S., Dulak-Lis M.G. Adipocyte-specific mineralocorticoid receptor overexpression in mice is associated with metabolic syndrome and vascular dysfunction: role of redox-sensitive PKG-1 and Rho kinase. Diabetes. 2016;65(8):2392–2403. doi: 10.2337/db15-1627. [DOI] [PubMed] [Google Scholar]
- 29.Urbanet R., Nguyen Dinh Cat A., Feraco A., Venteclef N., El Mogrhabi S., Sierra-Ramos C. Adipocyte mineralocorticoid receptor activation leads to metabolic syndrome and induction of prostaglandin D2 synthase. Hypertension. 2015;66(1):149–157. doi: 10.1161/HYPERTENSIONAHA.114.04981. [DOI] [PubMed] [Google Scholar]
- 30.Hirata A., Maeda N., Hiuge A., Hibuse T., Fujita K., Okada T. Blockade of mineralocorticoid receptor reverses adipocyte dysfunction and insulin resistance in obese mice. Cardiovascular Research. 2009;84(1):164–172. doi: 10.1093/cvr/cvp191. [DOI] [PubMed] [Google Scholar]
- 31.Watson M.L., Baehr L.M., Reichardt H.M., Tuckermann J.P., Bodine S.C., Furlow J.D. A cell-autonomous role for the glucocorticoid receptor in skeletal muscle atrophy induced by systemic glucocorticoid exposure. American Journal of Physiology – Endocrinology And Metabolism. 2012;302(10):E1210–E1220. doi: 10.1152/ajpendo.00512.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hrabe de Angelis M., Nicholson G., Selloum M., White J.K., Morgan H., Ramirez-Solis R. Analysis of mammalian gene function through broad-based phenotypic screens across a consortium of mouse clinics. Nature Genetics. 2015;47(9):969–978. doi: 10.1038/ng.3360. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.