Competing interest statement
Conflict of interest: the authors declare no potential conflict of interest.
Abstract
Listeria monocytogenes
(L.m.) is an agent of serious foodborne illness. It is a major concern for the food industry, since microorganism, growing in biofilms is protected against cleaning and disinfection and is difficult to eradicate. Aim of this study was to develop a protocol to assess the ability of two flexible packaging materials, named HGP40 and GND35, submitted to corona discharge treatment, to limit the production of L.m. biofilm at 12±1°C. Two strains were selected for this study: L.m ATCC 7644 and L. m. EURL 12MOB098LM isolated from dairy products. Results suggest that both L.m. strains were able to form biofilm on packaging materials tested. The differences on HGP40 and GND35s treated and not treated surfaces were not statistically significant.
Introduction
Listeria monocytogenes
(L. m.) is a Gram-positive food-borne pathogen widely distributed in the environment and commonly associated to food industry (Djordjevic et al., 2002). It can persist within food processing environments for long periods, due to its ability to grow at wideranging temperatures and pH and to form biofilms (Beresford et al., 2001; Wong, 1998). L. m is responsible of listeriosis, one of the most important food-borne diseases and the most commonly implicated vehicles are ready-to-eat products contaminated during processing (Mead et al., 1999; Lianou et al., 2007). The elderly, newborns, immune-compromised people and pregnant women are considered to be at high risk to contract disease (Swaminathan et al., 2007). Therefore, the demand for new and more effective antimicrobial strategy to control or eliminate this pathogen in foods became more urgent such as the use of mitochondrial targeting peptides (Palmieri et al., 2016; Falcigno et al., 2016). On the other hand, physical treatment of packaging material could be represented an alternative strategy. Corona discharge treatment is widely used in industry such as adhesive bonding, printing, extrusion coating, composite and heat-sealing (Brewis et al., 1981; Zhang et al., 1998). This treatment results in an increase in surface energy by introduction of polar groups on the surface, thus improving their adhesion and wetting properties (Gernenser et al., 1985; Mangipudi et al., 1995). However, little is known about the effects on subsequent microbial colonization and degradation (Guzel-Seydim et al., 2004).
Aim of the study was to assess the effect of corona treatment applied to two flexible packaging materials on L.m. biofilm formation by means of a specific protocol. The assay was carried out at the incubation temperature of 12±1°C to reproduce storage conditions in food industry, meat and dairy product particularly; furthermore, to check L. m. ability to form biofilm at low temperatures according Di Bonaventura et al., 2008 incubation time was extended to one week.
Materials and Methods
Bacterial strains preparation
Two reference strains of L. m. were selected for this study: L. m. ATCC 7644 and L. m. EURL 12MOB098LM isolated from dairy product. Culture of two strains were prepared inoculating 50 mL of BHI (Brain Heart Infusion, Sigma-Aldrich Co. LLC., St. Louis, MO, USA) into broth and adapting by incubating at selected temperature of 12±1°C. The cultures were allowed to growth until exponential phase and concentration adjusted to reach a final concentration of about Log10 8 CFU mL-1.
Packaging materials preparation
The biofilm formation assays were conducted on two different packaging films used in food industry identified as HGP40 and GND35 normally sold for packaging of pasta (Treofan Italia spa, Battipaglia, Salerno Italia); the first is a multilayer biaxially oriented polypropylene white coex-truded cavitated film, with a very low density (0.7 g/cm3) lower than normal bi-oriented polypropylene film and an opaque appearance to the light; it is produced by adding carbon dioxide to one layer of the packaging material. This film provides excellent barrier properties to water vapor and to the light, good barrier properties to oxygen and flavors; is heat-sealable on both sides (weldability range 105-140°C). GND35 packaging is a transparent coextruded bioriented polypropylene film composed of a central layer (core), consisting of polypropylene homopolymer (very shiny material, transparent and bright, with excellent mechanical characteristics, not heat-sealable) and of two thin skins (1-2 microns) of copolymer polypropylene (slightly more opaque material, but heat-sealable with excellent slipperiness). The core influences mechanical characteristics and transparence of the film; the skins confer the heat-sealing properties and the slip in the friction on metal surfaces. The film has a low density (0.91 g/cm3). Both are flexible and have been previously undergone to a physical treatment, named corona effect, using a shaping electrode. The corona discharge apparatus was available at factory (Treofan Italy Spa, Battipaglia, Salerno, Italy) and was composed by a ceramic faced corona discharge head with two electrodes connected in parallel to a high frequency, high voltage supply. Treatment was done according manufacturer procedures; Power could be adjusted by altering the period of the high frequency cycle that was at zero volts. Peak voltage and times at peak voltage were unaffected. The samples carried by the transport belts passed through two 5 mm wide coronas at 1 m/s giving each part of the sample 0.01 seconds exposure at each pass. After treatment the flexible packaging materials was brought to the laboratory for analysis.
Film was cut using a steel mold so that all materials have the same shape and size. The steel mold had a spherical shape (20-mm-diameter) and four arms (0.7 mm × 0.3 mm), which have the purpose to anchor the packaging materials at the bottom of the plates used for the test (Figure 1).
Figure 1.

Cutting of flexible packaging materials with a steel mold.
The shaped substrates were washed, sterilized completely immersed in 10 % acetone and left in gentle shaking with rocking platform shaker (Stuart, Bibby Scientific Limited, Staffordshire, UK) at 10 rpm for 10 minutes at room temperature. After a short washing in sterile deionized water (milli Q Millipore Merck KGaA, Darmstadt, Germany), the substrates were completely immersed in ethanol 100% (Sigma-Aldrich, St. Louis, USA) and left again in rocking platform shaker (Stuart, Bibby Scientific Limited, Staffordshire, UK) at 10 rpm for 10 minutes. At the end, the packaging materials were washed in sterile deionized water, dried, packaged and sterilized by autoclaving at 121°C for 15 minutes. The shaped packaging materials were placed into tissue culture six wells plate, with flat bottom and lid (Falcon®, Thermo Fisher Scientific Inc., Massachusetts, USA); preformed steel rings (Stainless steel Aisi 304) were located over the arms of packaging materials in order to keep them lying and immobilized at the bottom of each well (Figure 2). Biofilm formation test was conducted following the method described below (Di Bonaventura et al., 2008).
Figure 2.
The shaped packaging materials are placed into six wells plates used for the test and immobilized using steel rings.
Inoculum standardization
The bacterial suspension of L. m. previously prepared in BHI Broth (Sigma-Aldrich Co. LLC. St. Louis, USA) and adapted at 12±1°C for 48±1 h, was centrifuged for 5 minutes in a Servall SS-1 centrifuge at 18.000 X g and subjected to three washes in 10 mL of phosphate-buffered saline (PBS pH 7.3, Thermo Fisher Scientific Inc., Massachusetts, USA). After washing, the bacterial suspension was diluted and resuspended in new BHI Broth to reach a final concentration of about 108 CFU/mL which corresponds to an optical density value = 0.125 (OD600 nm) measured by the means of a Eppendorf Spectrophotometer (Thomas Scientific, Swedesboro, NJ, USA).
Experiment sets and biofilm production assay
To set up biofilm formation assay a preliminary trial was performed on both HGP40 and GND35 packaging materials (treated and untreated), carried out in triplicate and repeated in one independent set of experiments for each substrate.
Based on the results of the first preliminary assay the complete biofilm formation tests were conducted only on HGP40 substrates (treated and untreated), carried out in triplicate and repeated in six independent sets of experiments. In each experiment set, the packaging material to test, was placed into four out of six wells plate, three of them were inoculated with 3 mL of standardized inoculum (3 positive wells) while one well was inoculated with 3 mL of sterile BHI Broth as negative control. Subsequently the plates were closed with lid and parafilm “M” (Sigma-Aldrich, St. Louis, USA) and incubated at 12±1°C for one week in static condition. Simultaneously, enumeration of L. m. in accordance with ISO 11290-2:98 method was performed in order to verify the concentration and purity of the standardized inoculum. At the end of the incubation, the liquid media containing the planktonic cells were removed and enumerated (Gernenser et al., 1985). The packaging materials were washed with deionized sterile water by dipping, for three consecutive times. Washed materials were then placed in a new six wells plate to dry in an incubator at 60±1°C for about 1 h.
At the end of the fixing phase, 3 mL of 0.2 % Crystal Violet (Panreac Quimica SAU, Barcelona, Spain) in 95 % ethanol (Sigma-Aldrich, St. Louis, MO, USA) solution were added to each well for staining the substrates and gently shaked for 15 minutes using rocking platform shaker (Stuart, Bibby Scientific Limited, Staffordshire, UK) at 10 rpm. After staining, packaging materials were washed three times by dipping in sterile deionized water, thus eliminating the Crystal Violet excess solution and transferred into a new plate. Plates were then incubated at 37±1°C until complete drying.
After drying, the arms of each support were cut and 3 mL of 33 % acetic acid (Sigma-Aldrich, St. Louis, USA) to destain the samples were added; then plates were left shaking for 15 minutes in rocking platform shaker (Stuart, Bibby Scientific Limited, Staffordshire, UK) at 10 rpm. The acetic acid solution from one well was collected and distributed in amounts of 200 microliters in 12 wells of a microtiter U plate (96 multiwell plate Laboindustria SpA, Arzergrande, PD, Italy). Then the plate was placed in a Spectrophotometer (Sunrise, Tecan Trading AG, Switzerland) to measure the absorbance of crystal violet present in destaining solution at a wavelength of 492 nm.
Scanning electron microscope of Listeria monocytogenes biofilm
Three-dimensional architecture of biofilm, developed on the packaging materials, was studied by scanning electron microscopy (SEM) technique (Zeiss Sigma microscope, Carl Zeiss, Oberkochen, Germany). All packaging materials were washed by dipping three times in sterile phosphate-buffered saline (PBS pH 7.3, Thermo Fisher Scientific Inc., MA, USA) to remove planktonic cells. To each sample 3 mL of Karnovsky’s Fixative (Electron Microscopy Sciences, Hatfield, PA, USA) were added and placed at 4±1°C for 1 h. Then samples were washed in 3 mL of 0.1 M sodium cacodylate buffer (C4945 SIGMA) CAS Number 6131-99-3, pH 7.4 for 15 minutes for four times.
Statistical analysis
All preliminary experiments on both GND35 and HGP40 treated and untreated packaging materials were carried out in triplicate in one independent set of experiments. Optical Density (OD) values were compared through Mann-Whitney non parametric test for independent samples for preliminary date on GND35 and HGP40. All subsequent experiments were carried out in triplicate only on HGP40 treated and untreated packaging materials. OD values were compared through non-parametric Mann-Whitney test for comparison between two independent groups (treated HGP40 and untreated HGP40) processed in different period of time (six independent set of experiments). Statistical analysis were performed using Microsoft® Excel 2000/XLSTAT©-Pro (Version 7.2, 2013.2.04, Addinsoft, Inc., Brooklyn, NY, USA).
Results
Biofilm production: crystal violet based colorimetric method
Biofilm was produced on both packaging materials at 12±1°C. Result of preliminary trial showed that the absorbance values were 0.041 (0.0173 to 0.0593) for GND35 treated and 0.038 (0.0231 to 0.0621) for untreated GND35 surfaces. Difference between averages was 0.004 and the P=0.107 indicate a not statistically significant difference (P>0.10) in the ability of L.m. strains to form biofilm at 12±1°C on treated and untreated GND35 packaging material (Figure 3).
Figure 3.
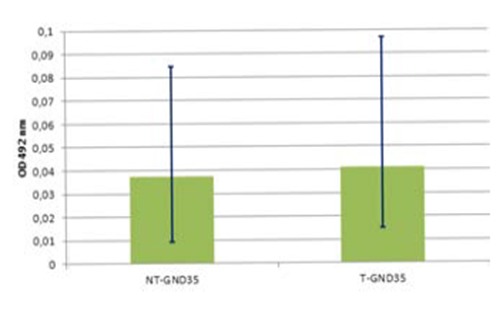
Biofilm formation by Listeria monocytogenes strains at 12±1°C on GND35 surfaces. Mann-Whitney test (P=0.107) shows not significant statistical differences on GND35 treated (T-GND35) and untreated (NT-GND35) packaging materials.
Concerning HGP40 the absorbance values were 0.015 (0.0081 to 0.0271) for treated HGP40 surfacesand 0.028 (0.0158 to 0.0498) for untreated HGP40 surfaces. Difference between averages was 0,013 and the P<0.0001, indicate a statistically significant difference (Figure 4). Complete biofilm formation assays were performed only on HGP40, 432 results were obtained by CV-OD492 measurement (216 values for treated HGP40 and 216 values for untreated HGP40). Calculated absorbance average on HGP40 treated surfaces was 0.022 (0.002 to 0.081) and on HGP40 untreated surfaces was 0.021 (0.003 to 0.051). The difference between averages was 0.001 and the P value=0.173 indicate a not statistically significant difference (Figure 5).
Figure 4.
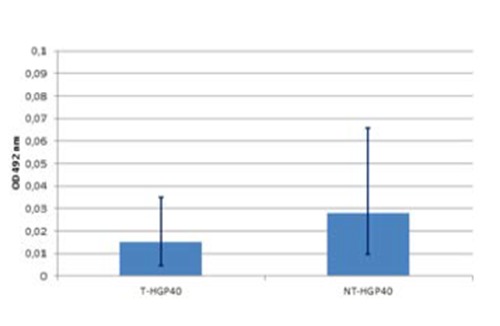
Biofilm formation by Listeria monocytogenes strains at 12±1°C on HGP40 surfaces. Mann-Whitney test (P<0.0001) indicates that biofilm formation on untreated surfaces (NT-HGP40) is significantly higher than biofilm formation on treated surfaces (T-HGP40).
Figure 5.
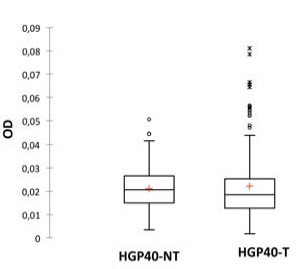
Biofilm formation by Listeria monocytogenes strains at 12±1°C on HGP40 surfaces. Mann-Whitney test (P=0.173) indicates that biofilm formation on untreated surfaces (NT-HGP40) is not statistically significant different from biofilm formation on treated surfaces (T-HGP40).
Scanning electron microscope analysis of Listeria monocytogenes biofilms
The protocol developed has proved to be effective for highlighting L. m. biofilm formation on selected surfaces. A rudimentary biofilm consisting only of sparse clusters of cells and minimum amounts of extracellular polymeric substance (EPS) was produced on both packaging materials (treated and untreated) at 12±1°C (Figure 6).
Figure 6.
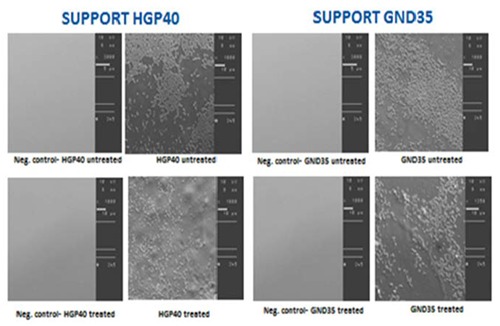
Scanning electronic microscope images of biofilm formed by Listeria monocytogenes on packaging material support HGP40 (treated and untreated) and GND35 (treated and untreated). Magnification: 1000x.
Discussion
Results of this study suggest that L. m. was able to form biofilm on all the tested packaging materials at 12±1°C. However, based on the SEM results, L. m. strains did not exhibit a complex organization and architecture. Scattered aggregates of cells and minimum amounts of EPS are detected, probably because of a less advanced state of growth rate rather than to a different cellular physiology although the incubation time was extended to one week (Di Bonaventura et al., 2008).
It is important to underline that trial was conducted at 12±1°C because it is the temperature commonly used to check shelf life of food products as reported in the European Union Reference Laboratory for Listeria monocytogenes (2014) technical guidance. Moreover, the above-mentioned temperature is normally used in food industry during processing and it is considered as indicator of abuse temperature during transport and food storage at retail and at domestic level.
Results of the preliminary tests showed no difference between GND35 treated and untreated surfaces. Differently from GND35, the treated surfaces of HGP40 showed a statistically significant (P<0.0001) reduction of biofilm formation produced by L. m. compared to the untreated surface at 12±1°C.
A possible mechanism of chemical reactions caused by corona discharge treatment on packaging materials should be due to an increased surface energy following the treatment; Oxygen molecules are activated by the energy from the corona and elemental oxygen (O), activated oxygen molecule (O2) and/or ozone (O3) are created. After a very short period of time, the activated oxygen reacts with the activated surface to form a stable oxidised surface. Enrichment of the surface energy due to the formation of oxidised compounds as well as that of free radicals on the surface appears to have taken place (Gernenser et al., 1985).
To validate preliminary results on HGP40 further tests were performed. Results showed that differences on HGP40 treated and not treated surfaces were not statistically significant.
Conclusions
The aim of this study was to investigate the ability of a physical treatment to limit biofilm formation on food packaging grade polypropylene films. In conclusion, considering complete biofilm assay, the corona discharge treatment seems to not have effect in the overall rate of biofilm formation by L. m. on both packaging material tested. Further studies are needed to determine if different parameters of Corona treatment could have a significant effect.
Acknowledgements
The authors thank Treofan Italia spa (IT) for the loan of the corona discharge head used for film treatment.
References
- Beresford MR, Andrew PW, Shama G, 2001. Listeria monocytogenes adheres to many materials found in food-processing environments. J Appl Microbiol 90:1000-5. [DOI] [PubMed] [Google Scholar]
- Brewis DM, Briggs D, 1981. Adhesion to polyethylene and polypropylene. Polymer 22:7-16. [Google Scholar]
- Di Bonaventura G, Piccolomini R, Paludi D, D'Orio V, Vergara A, Conter M, Ianieri A, 2008. Influence of temperature on biofilm formation by Listeria monocytogenes on various food-contact surfaces: relationship with motility and cell surface hydrophobicity. J Appl Microbiol 104:1552–61. [DOI] [PubMed] [Google Scholar]
- Djordjevic D, Wiedmann M, McLandsborough LA, 2002. Microtiter plate assay for assessment of Listeria monocytogenes biofilm formation. Appl Environ Microb 68:2950-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- European Union Reference Laboratory for Listeria monocytogenes, 2014. EURL Lm technical guidance document for conducting shelf-life studies on Listeria monocytogenes in ready-to-eat foods. Available from: https://sites.anses.fr/en/system/files/private/LIS-Cr-201403D1.pdf [Google Scholar]
- Falcigno L, Palmieri G, Balestrieri M, Proroga YTR, Facchiano A, Riccio A, Capuano F, Marrone R, Campanile G, Anastasio A, 2016. NMR and computational data of two novel antimicrobial peptides. Data Brief 8:562-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gernenser LJ, Elman JF, Mason MG, Pochan JM, 1985. E.s.c.a. studies of corona-discharge-treated polyethylene surfaces by use of gas-phase derivatization. Polymer 26:1162. [Google Scholar]
- Guzel-Seydim ZB, Greene AK, Seydim AC, 2004. Use of ozone in the food industry. LWT - Food Sci Technol 37:453-60. [Google Scholar]
- ISO, 1998. Microbiology of food and animal feeding stuffs-- Horizontal method for the detection and enumeration of Listeria monocytogenes-- Part 2: Enumeration method. ISO norm 11290-2:1998. International Standardization Organization, Geneva, Switzerland. [Google Scholar]
- Lianou A, Sofos JN, 2007. A review of the incidence and transmission of Listeria monocytogenes in ready-to-eat products in retail and food service environments. J Food Protect 70:2172-98. [DOI] [PubMed] [Google Scholar]
- Mangipudi V, Tirrell M, Pocius VA, 1995. Direct measurement of the surface energy of corona-treated polyethylene using the surface forces apparatus. Langmuir 11:19-23. [Google Scholar]
- Mead PS, Slutsker L, Dietz V, Mc Caig LF, Bresee JS, Shapiro C, Griffin PM, Tauxe RV, 1999. Food-related illness and death in the United States. Emerg Infect Dis J 5:607-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmieri G, Balestrieri M, Proroga Y, Falcigno L, Facchiano A, Riccio A, Capuano F, Marrone R, Neglia G, Anastasio A, 2016. New antimicrobial peptides against foodborne pathogens: From in silico design to experimental evidence. Food Chem 211:546-54. [DOI] [PubMed] [Google Scholar]
- Swaminathan B, Gerner-Smidt P, 2007. The epidemiology of human listeriosis. Microbes Infection 9:1236-43. [DOI] [PubMed] [Google Scholar]
- Wong AC, 1998. Biofilms in food processing environments. J Dairy Sci 81:2765-70. [DOI] [PubMed] [Google Scholar]
- Zhang D, Sun Q, Wadsworth LC, 1998. Mechanism of corona treatment onpolyolefin films. Polymer Eng Sci 38:965-70. [Google Scholar]


