Figure 2.
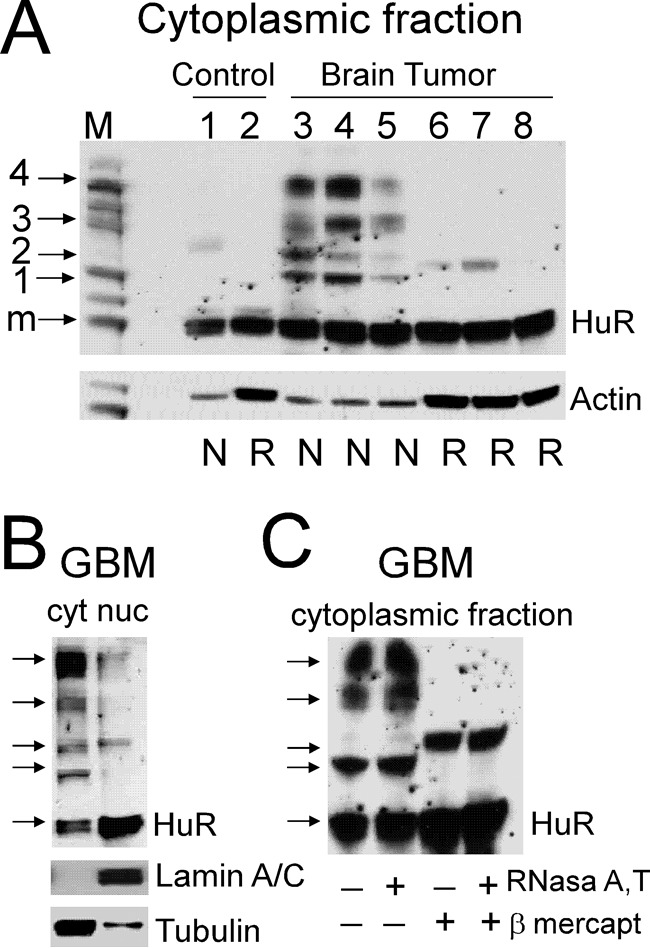
Assessment of HuR protein multimerization in control and brain tumor samples by Western blotting. A, examples of HuR protein dimerization/multimerization detected in non-reducing and non-denaturing conditions (N) versus reducing and denaturing conditions (R) in control and tumor samples. Note that lanes 1 and 2, 3 and 6, 4 and 7, and 5 and 8 represent proteins from the same samples at two different conditions. Each lane was loaded with an equal amount of protein. The actin level served to confirm equal loading. Note that the actin antibody has differing ability to recognize actin in the non-denatured, non-reduced condition compared with the denatured, reduced condition. The protein ladder is shown in lane M; the arrows correspond to 30 (monomeric HuR; m), 50 (1), 60 (2), 90 (3), and 120 kDa (4) molecular masses. B, examples of HuR protein multimerization in the non-reduced and non-denatured condition in cytoplasmic (cyt) and nuclear (nuc) fractions of the tumor sample. Lamin A/C and α-tubulin antibodies were used to confirm nuclear and cytoplasmic fractions, respectively. C, examples of HuR protein dimerization/multimerization from the same tumor sample in four different conditions: non-denatured and non-reduced (lane 1), pretreated with RNase A/T (lane 2), pretreated with β-mercaptoethanol (lane 3), and pretreated with both RNase A/T and β-mercaptoethanol (lane 4).
