Figure 3.
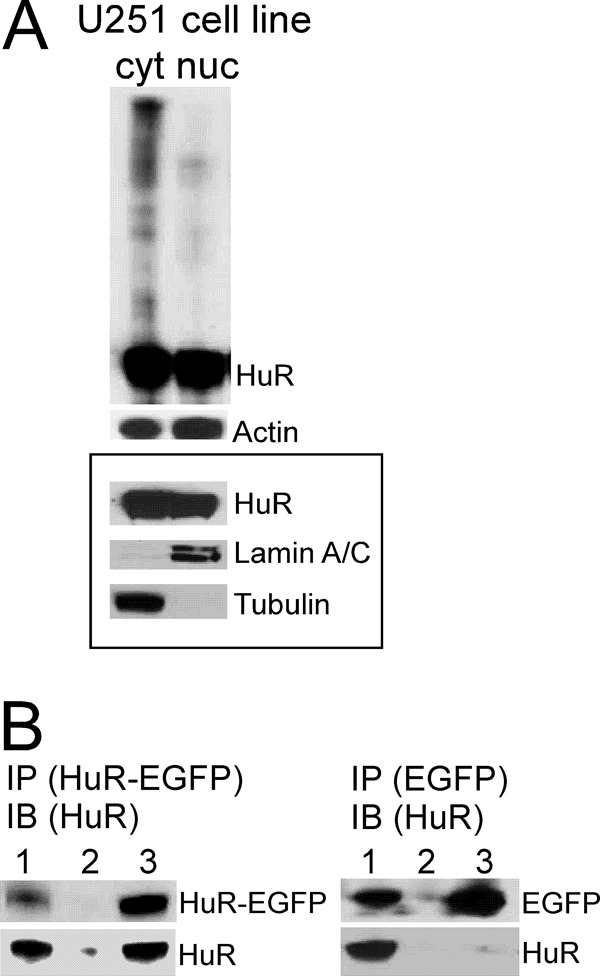
Assessment of HuR protein multimerization in protein samples from U251 glioma cell line. A, example of HuR protein multimerization in the non-reduced and non-denatured condition in cytoplasmic (cyt) and nuclear (nuc) fractions detected by Western blotting. Actin served to confirm equal protein loading. The framed inset represents HuR distribution in nuclear and cytoplasmic fractions in the reduced and denatured condition. Lamin A/C and α-tubulin antibodies were used to confirm nuclear and cytoplasmic fractions, respectively. B, example of coimmunoprecipitation of endogenous HuR protein (30 kDa) with HuR-EGFP protein (50 kDa) transfected in the U251 cell line and with EGFP (20 kDa) as a control. Immunoprecipitation (IP) of HuR-EGFP protein or EGFP was performed using EGFP antibody; immunoblotting (IB) was performed using HuR3A2 antibody. The lanes correspond to input (lane 1), immunoprecipitation using rabbit IgG antibody (lane 2), and immunoprecipitation using EGFP antibody (lane 3).
