Figure 8.
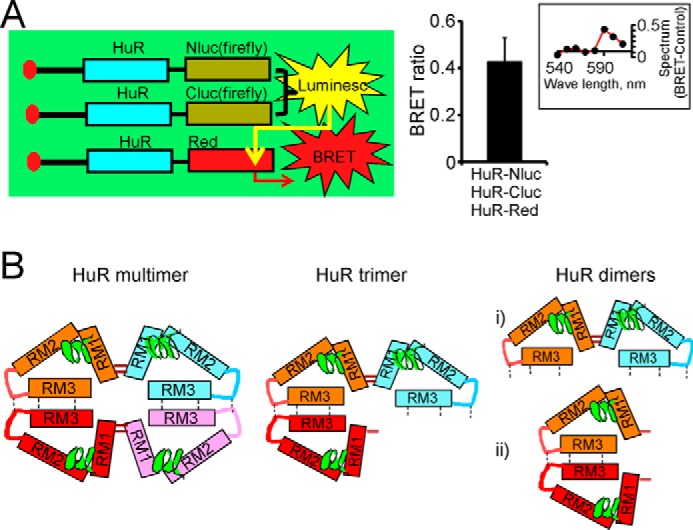
Confirmation of HuR multimerization by combined split firefly luciferase and BRET assays. A, schematic representation demonstrating detection of HuR multimerization in combined split firefly luciferase and BRET assays (left). The graph provides averaged BRET ratio detected between firefly luciferase reconstituted during HuR dimerization in split luciferase assay and HuR-DsRed fluorescence construct coexpressed in the same cells (see “Experimental procedures” for details). The error bars represent S.D. d-Luciferin was used as a firefly luciferase substrate. The inset illustrates the difference between the superimposed and normalized emission spectra of BRET condition (from cells coexpressing HuR-Nluc, HuR-Cluc, and HuR-DsRed constructs) and the control condition (cells coexpressing HuR-Nluc, HuR-Cluc, and DsRed constructs). The spectrum difference is presented from 540 to 610 nm with 10-nm individual measurement interval. The averaged ratio of the signal at 590 nm to the signal at 570 nm was 0.81 ± 0.1 (n = 4) for control and 1.22 ± 0.1 (n = 4) for BRET condition. The expression of endogenous HuR was decreased by using siHuR against HuR 3′-UTR (see “Experimental procedures” for details). B, potential models of HuR multimerization and dimerization. The HuR tetramer and trimer models (left and center, respectively) include concurrent disulfide bond formation between HuR N termini and hydrophobic oligomerization of hinge and RRM3 domains. HuR dimerization models (right) rely on formation of a disulfide bond between HuR N termini or oligomerization of hinge and RRM3 domains.
