Figure 4.
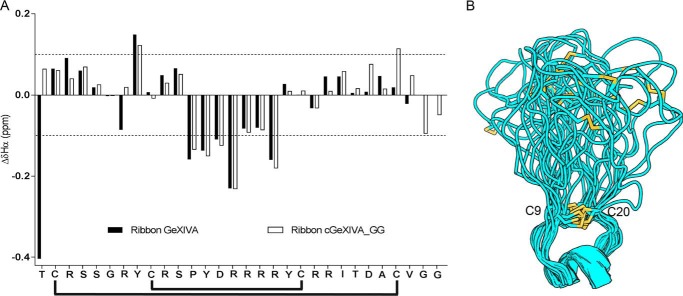
Structural characterization of cGeXIVA_GG. A, secondary αH chemical shifts of ribbon GeXIVA and ribbon cGeXIVA_GG (ΔδHα). The overall secondary structures of ribbon GeXIVA and ribbon cGeXIVA_GG are similar, and both peptides display a 310-helix from Pro12 to Arg16. The black lines below the sequence show the ribbon disulfide connectivity. B, ribbon diagram of the NMR solution structure of cGeXIVA_GG. Models were overlaid over the Cys9–Cys20 segment. Disulfide bonds are shown in yellow.