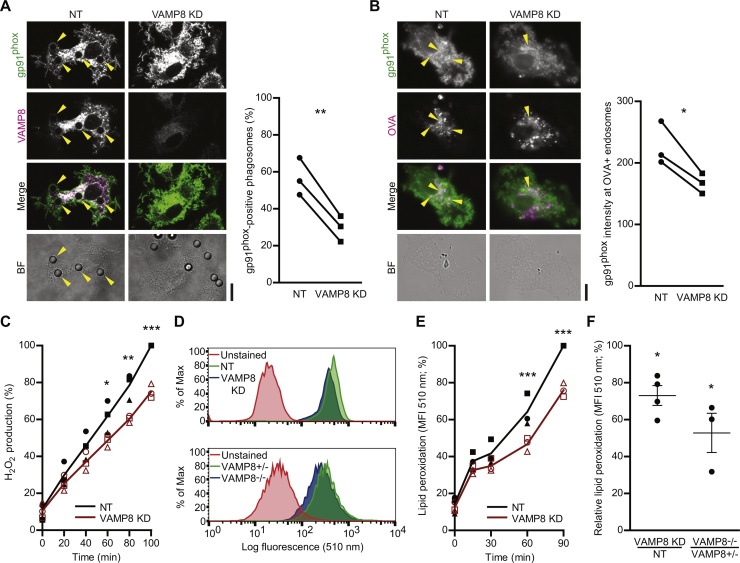
Fig. 2.
VAMP8 is responsible for NOX2 recruitment to endosomes and phagosomes. (A) Representative confocal micrographs showing the recruitment of gp91phox (green in merge) to phagosomes containing latex beads by immunofluorescence in VAMP8 KD DCs and control DCs (non-targeting siRNA; NT). Magenta: immunolabeling for VAMP8. Arrow heads indicate phagosomes positive for gp91phox. The graph shows the quantification by manual counting of gp91phox-positive phagosomes of DCs from 3 individual donors (linked by solid lines; p = 0.0067). (B) Same as panel A, but now for ovalbumin-containing endosomes (OVA; magenta; p = 0.0391). Recruitment was quantified from the gp91phox signal at OVA-positive compartments relative to the total imaged cell area. (C) H2O2 production by VAMP8 KD DCs (red curve) compared to NT control DCs (black) measured with the Amplex Red assay. The symbols show the individual donors. (D) Representative flow cytometry histograms of lipid peroxidation sensor BODIPY581/591-C11 for VAMP8 KD (blue) and NT (green) human DCs (upper graph) and for VAMP8−/− (blue) and VAMP8± (green) mouse BMDCs (lower graph) after 60 min incubation with LPS and OVA. The red curves show the background fluorescence distribution from cells without BODIPY581/591-C11. (E) Percentage of lipid peroxidation over time in VAMP8 KD (red curve) and NT (black) human DCs. The symbols show the individual donors. (F) Quantification of panel D. Relative lipid peroxidation: mean fluorescence intensities of VAMP8 KD DCs (4 donors; p = 0.0154) and VAMP8-/- BMDCs (3 mice; p = 0.0474) relative to the NT and VAMP8± controls. Scale bars, 10 μm. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)