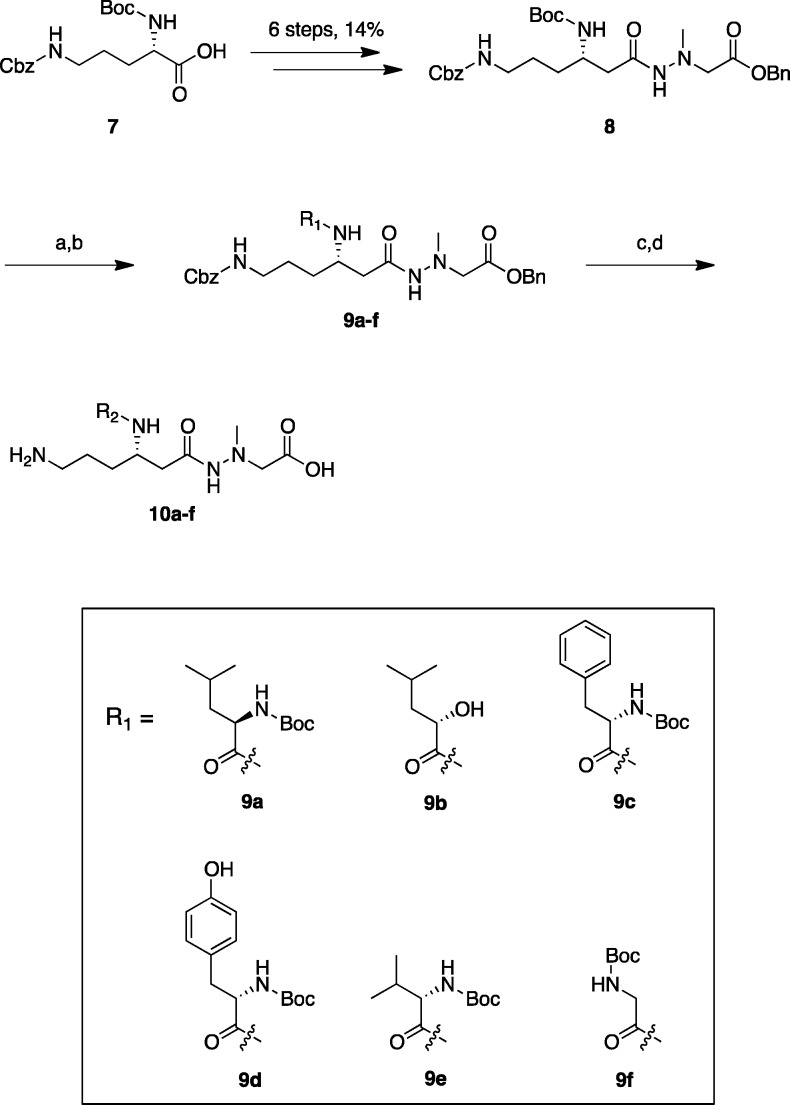
Scheme 1. Synthesis of Derivatives 10a–f.
Reagents and conditions: (a) 4 M HCl/dioxane, rt, 1 h; (b) Boc-protected amino acid (for 9a, 9c–f) or (S)-2-hydroxy pentanoic acid (for 9b), EDC·HCl, HOBt·H2O, Et3N, DMF, rt, overnight, 50–95% (2 steps); (c) H2, Pd/C, MeOH, rt, 3 h, quant, then RP-HPLC, 65% (for 10b); (d) 4 M HCl/dioxane, rt, 1 h, then RP-HPLC, 30–47% (for 10a, c–f). R2 substituents of derivatives 10a–f are shown in Table 1.