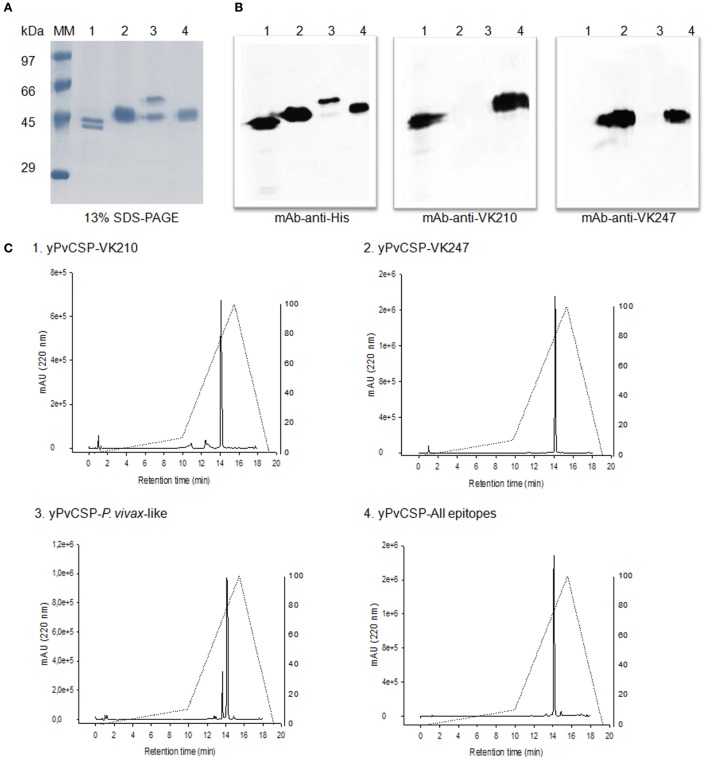
Figure 2.
Purification and biochemical characterization of the Plasmodium vivax circumsporozoite protein (CSP) recombinant proteins. (A) 13% SDS-PAGE under reduced conditions stained with Coomassie blue. Proteins migrate between 50 and 55 kDa. Fractions were 2 µg of: (1) yPvCSP-VK210, (2) yPvCSP-VK247, (3) yPvCSP-P. vivax-like, and (4) yPvCSP-All epitopes. (B) Western blot using 1 µg of the same fractions of proteins described above. Antibodies used were monoclonal antibody (MAb) anti-His, MAb VK210 (2F2), and MAb VK247 (2E10.E9). The secondary antibody used was anti-mouse IgG HRP-labeled, and detection was performed by ECL assay. (C) The purity of proteins, after the combination of chromatographic methods, was analyzed by reverse-phase high-performance liquid chromatography (RP-HPLC), in which the gradient elution was developed combining 0.1% trifluoroacetic acid (TFA) in water and 0.1% TFA in 90% acetonitrile, 24°C, 1 mL/min for 40 min, in a C18 column.