Abstract
Objective
Obsessive-compulsive disorder (OCD) is common and debilitating with patients exhibiting persistent intrusive thoughts (obsessions), repetitive ritualistic behaviours (compulsions) and anxiety. While it is known that OCD is highly heritable, the specific genetic risk factors for OCD are still largely unknown. The etiology of OCD has also not been fully elucidated but there is growing evidence that glutamate signaling dysfunction in the cortico-striatal-thalamo-cortical (CSTC) circuitry plays a role in its pathogenesis.
Methods
We conducted a focused review of recent literature on the role of glutamate genes in OCD.
Results
There have been several recent discoveries in the SAPAP (DLGAP) family, SLC1A1, and GRIN/GRIK families of proteins related to OCD.
Conclusion
There is growing evidence supporting a role for genetic variation leading to dysfunctional glutamate signaling in OCD. Based on this new evidence we hypothesize that sustained glutamatergic neurotransmission in key areas of the brain may be contributing to the etiology of OCD.
Keywords: OCD, glutamate, genetics
Résumé
Objectif
Le trouble obsessionnel-compulsif (TOC) est commun et débilitant chez les patients qui présentent des pensées intrusives (obsessions) persistantes, des comportements rituels répétitifs (compulsions) et de l’anxiété. Bien que l’on sache que le TOC est fortement héréditaire, les facteurs de risque génétique spécifiques du TOC sont encore largement inconnus. L’étiologie du TOC n’a pas non plus été encore pleinement élucidée, mais il apparaît de plus en plus que le glutamate qui signale une dysfonction dans le circuit cortico-striatal-thalamo-cortical (CSTC) joue un rôle dans sa pathogenèse.
Méthodes
Nous avons mené une revue ciblée de la littérature récente sur le rôle des gènes du glutamate dans le TOC.
Résultats
Il y a eu plusieurs découvertes récentes dans la famille SAPAP (DLGAP), les familles de protéines SLC1A1, et GRIN/GRIK liées au TOC.
Conclusion
Les preuves s’accumulent à l’appui du rôle d’une variation génétique menant à un signal dysfonctionnel du glutamate dans le TOC. Selon ces nouvelles preuves, nous émettons l’hypothèse que la neurotransmission glutamatergique soutenue dans les principales régions du cerveau peut contribuer à l’étiologie du TOC.
Mots clés: TOC, glutamate, génétique
Patients with obsessive-compulsive disorder (OCD) exhibit unwanted repeated thoughts and repetitive behaviours. The World Health Organization placed OCD among the ten most disabling conditions worldwide (Murray & Lopez, 1996), with a lifetime prevalence of 1–3% (Kessler et al., 2005; Valleni-Basile et al., 1994). OCD is highly heritable (Hanna, Himle, Curtis, & Gillespie, 2005; van Grootheest, Cath, Beekman, & Boomsma, 2005), however the specific genetic risk factors for OCD are still largely unknown.
Early research focused on serotonin as the major neurotransmitter involved in the pathogenesis of OCD because of the efficacy of serotonin reuptake inhibitors in treatment of the disorder. In contrast, the glutamate hypothesis of OCD was first proposed based on imaging and other biological studies before glutamate agents were tested (Rosenberg & Keshavan, 1998). While we (K. Wu et al., 2012) and others (Pittenger, Bloch, & Williams, 2011) have previously reviewed the role of glutamate in OCD, this review will focus on new developments in genes associated with OCD supported by multiple levels of analysis (i.e., genetics, animal models, and imaging). This review will focus on new findings in glutamate related synapse (SAPAP/DLGAP family), transporter (SLC1A1) and NMDA receptor genes (GRIN and GRIK family). These findings further implicate glutamate in OCD and suggest a narrative explaining how these glutamate system genes may lead to obsessive-compulsive symptoms.
SAPAP family in Mouse Models
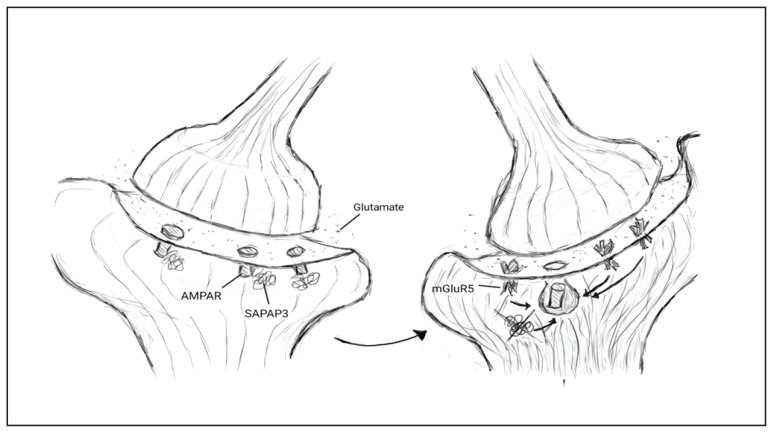
The gene family with the strongest evidence supporting the role of glutamate in OCD through both mouse models and human genetic studies is the SAPAP or DLGAP (in humans) family of proteins. SAPAPs are a family of membrane-associated guanylate kinases that form scaffolding complexes which regulate the trafficking and targeting of neurotransmitters to the post-synaptic membrane during synaptic transmission. SAPAP3 is the only member of this family that is highly expressed in the striatum, a region implicated in OCD etiology (Harrison et al., 2009; Welch et al., 2007). SAPAP3 may alter glutamate signalling via α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors (AMPAR) and Group 1 metabolic glutamate receptors (mGluRs) (Figure 1).
Figure 1.
A pictorial depiction of the relationship between AMPA receptors (AMPAR), SAPAP3, and mGluR5. Knocking out SAPAP3 results in endocytosis of AMPARs and upregulation of mGluR5 receptors which further promotes AMPAR endocytosis. This change to fewer fast acting glutamate receptors (AMPAR) and more slow but sustained glutamate receptors (mGluR5) results in OCD-like behaviour in mice.
Knocking-out SAPAP3 in mice produces behavioural and neural features relevant to OCD. SAPAP3 knockout-mice excessively groom, show anxiety-like behaviour, and treatment with selective serotonin reuptake inhibitors (SSRIs) reduce these behaviors (Welch et al., 2007). SAPAP3-knockout mice also show abnormalities at cortico-striatal synapses. Selective expression of SAPAP3 in the striatum rescued these synaptic and behavioral anomalies. A recent optogenetic study of SAPAP3 knockout-mice implicated the lateral orbito-fronto-striatal pathway in repetitive behavior (Burguière, Monteiro, Feng, & Graybiel, 2013). Selective optogenetic stimulation of this pathway in SAPAP3 knockout-mice restored inhibitory signaling and prevented overexpression of conditioned and spontaneous repetitive grooming.
One possible biological mechanism which may mediate the effects of SAPAP3 in OCD-like behavior in mice is AMPARs. AMPARs are the most ubiquitous glutamate receptors in the nervous system and contribute prominently to excitatory neurotransmission by mediating the fast, rapidly desensitizing excitation of synapses (Zarate & Manji, 2008). SAPAP3 knockout-mice showed less AMPAR mediated transmission in striatal inhibitory medium spiny neurons (MSNs) (Wan, Feng, & Calakos, 2011); the most prevalent neuron-type in the corpus striatum of the basal ganglia. The AMPAR-mediated transmission in MSNs in SAPAP3 knockout-mice was reduced by postsynaptic endocytosis of AMPARs, not structural loss or decrease in presynaptic release probability (Wan et al., 2011).
mGluRs are also altered in SAPAP3 knock-out mice. These receptors were more active and prevalent on the dendrites of striatal MSNs of SAPAP3 knockout-mice than wild-type MSNs (Chen et al., 2011). Antagonism of mGluR5 receptors reversed the reduction in AMPAR synaptic transmission in SAPAP3 knock-out MSNs (Chen et al., 2011). In the MSNs of wild-type mice, post-synaptic AMPAR activity was reduced in response to a mGluR5-selective positive allosteric modulator. Thus, in SAPAP3 knockout-mice high levels of mGluR5 signaling may silence postsynaptic AMPAR synapses through endocytosis (Wan et al., 2011) (Figure 1). These findings provide strong evidence that at least two glutamate receptor types (AMPARs and mGluRs) interact with the SAPAP3 protein. Furthermore, synthesizing what was found in the above articles, it appears that in SAPAP3 knockout mice there is a shift to fewer fast acting glutamate receptors (AMPAR) and more slow acting receptors (mGluR5) resulting in a more sustained response to glutamate (Figure 1). We hypothesize that a similar mechanism may be involved in the etiology of OCD in humans.
Human Genetics Findings in the DLGAP (SAPAP) family
DLGAP3 is the human homolog of SAPAP3, and has been associated with conditions which affect the brain such as schizophrenia and Tourette’s syndrome (Crane et al., 2011; Li et al., 2013). Despite consistent evidence linking SAPAP3 to OCD-like phenotypes in mice, findings from candidate gene association studies of DLGAP3 have been mixed. A family-based association study of 383 families found no single nucleotide polymorphism (SNP) or haplotype within DLGAP3 to be associated with OCD, although a few SNPs were nominally associated (P<0.05; uncorrected) with trichotillomania (TTM) and similar disorders (nail biting; excoriation) (Bienvenu et al., 2009). These grooming disorders are phenomenologically and etiologically related to OCD and were included in the category of Obsessive-Compulsive and Related Disorders in the DSM-V (Association, 2013; Monzani, Rijsdijk, Harris, & Mataix-Cols, 2014). Boardman et al. (2011) genotyped 7 polymorphic variants across DLGAP3 in South African white OCD (n=172), TTM (n=45), and control (n=153) subjects. Single-locus analysis revealed that rs11583978 was significantly associated with DLGAP3 and TTM, though the significance was lost after correction for multiple testing. In the OCD group, an earlier age of onset was significantly associated with a specific DLGAP3 haplotype (rs11583978-rs7541937-rs6662980-rs4652867).
While human studies of DLGAP3 have been mixed, the first published genome-wide association study in OCD (GWASs) has suggested another member of this gene family, DLGAP1 may be involved in OCD. In this GWAS, which included 1465 cases, 5557 ancestry-matched controls and 400 trios, the two lowest p-values in the case-control sub-analysis were of SNPs in DLGAP1 (P=2.49×10-6 and P=3.44×10-6). A second published GWAS in a sample of childhood-onset patients did not detect any SNP to be associated with OCD at the genome-wide significance level (Mattheisen et al., 2014).
GWASs are designed to detect common polymorphisms, not rare variants which are best detected using sequencing methods. Rare variants in DLGAP3 could have deleterious effects, as seen in the mouse knockout models, if the variants are present in conserved coding regions of the gene. Züchner et al. (2009) fully re-sequenced DLGAP3 in patients with OCD and TTM, identifying 7 novel non-synonymous heterozygous variants. A pooled analysis of these rare variants revealed that a significantly greater proportion of OCD/TTM patients compared to controls had at least one rare variant (4.2% vs. 1.1%, respectively). These variants have yet to be screened in a larger OCD cohort. A recent study also re-sequenced exonic regions of DLGAP3 in 215 patients with schizophrenia, a disorder highly comorbid with OCD in which glutamatergic pathways are similarly strongly implicated (Li et al., 2013; Schirmbeck & Zink, 2013). In this study, patient and control groups carried a similar proportion of missense mutations. Functional studies are required to determine the biological significance of the DLGAP3 rare variants and whether their effects are commensurate to those observed in SAPAP3 knock-out mice.
Human Genetic Findings in a Glutamate Transporter Gene (SLC1A1)
The 9p24 region first showed suggestive linkage in 7 large families with OCD (Hanna et al., 2002). This finding was independently replicated by another linkage study focused solely on the 9p24 region in 50 pedigrees with OCD (Willour et al., 2004). Within this region there are approximately 50 gene families. While several of the genes in the region are expressed in the CNS, only two genes code for proteins that have been shown to interact with glutamate; SLC1A1 and PTPRD. Furthermore, SLC1A1 is a post-synaptic glutamate transporter and the only gene most consistently associated with OCD in human studies. Five independent family based-association studies (Arnold, Sicard, Burroughs, Richter, & Kennedy, 2006; Dickel et al., 2006; Samuels et al., 2011; Shugart et al., 2009; Stewart et al., 2007) and one case-control study (Wendland et al., 2009) have found that alleles within SLC1A1 are associated with OCD. A recent study that analyzed four SLC1A1 SNPs in 244 early-onset OCD cases, 244 late-onset cases, and 244 healthy controls from a Han Chinese population, detected differences in allele and genotype frequencies of one SNP (rs10491734) between early-onset and late-onset OCD patients (H. Wu et al., 2013). Furthermore, a four-locus haplotype was associated with early-onset OCD after Bonferroni correction. While most of the observed associations are towards the 3′ region of the gene, specific allele associations have not been consistently replicated across studies. The second OCD GWAS adds to the heterogeneity of the results; the SNP with the lowest p-value was on chromosome 9 near PTPRD (Receptor-type tyrosine-protein phosphatase delta; P= 4.13 ×10-7) (Mattheisen et al., 2014). PTPRD itself has been linked to increased differentiation of glutamatergic synpases and it lies on the same linkage peak as SLC1A1; as identified in previous studies using extended pedigrees with OCD (Hanna et al., 2002; Kwon, Woo, Kim, Kim, & Kim, 2010).
To clarify the association between SLC1A1 and OCD, our group conducted a meta-analysis of the nine previously associated SLC1A1 SNPs in 815 trios, 306 cases and 634 ethnicity- and sex-matched controls which were primarily Caucasian (Stewart et al., 2013). One SLC1A1 SNP was weakly associated with OCD (rs301443, p=0.046 non-significant when corrected), and another SNP was modestly associated with OCD only in males (P=0.012). The lack of clear association with common variants could be due to small effect size, phenotypic and/or genetic heterogeneity, or the presence of untested causal rare variants. Larger samples, analysis of distinct clinical ‘subtypes’ of OCD in future association studies, and analysis of a full spectrum of common and rare variants using sequencing methods may help clarify this.
Patients with OCD may also have rare variants in SLC1A1. Rare variants identified in OCD patients include a rare 11 bp deletion located just downstream of SLC1A1 (Dickel et al., 2006) and a single rare SNP (Ala164) identified through mutation screening of over 300 OCD patients (0.14%, 1/738 chromosomes) (Wang et al., 2010). In a study of dicarboxylic aminoaciduria, a rare autosomal recessive renal disorder (Bailey et al., 2011), a proband with obsessive-compulsive symptoms harbored a coding mutation in exon 12 of SLC1A1. This variant is in close proximity to common variants previously identified in earlier studies of OCD (Arnold et al., 2006; Wendland et al., 2009). Our group screened 184 males with OCD for rare variants in SLC1A1 exons; however no new coding variation was found (Veenstra-VanderWeele et al., 2012). Transfection of the Ala164 missense variant into human embryonic immortalized kidney cells revealed a statistically significant decrease in glutamate transport (Veenstra-VanderWeele et al., 2012). These earlier studies didn’t comprehensively sequence SLC1A1 using next-generation sequencing (NGS) methods, but rather used older mutation screening methods which may have missed variants. NGS may help identify additional rare variants of SLC1A1 in patients with OCD. Our group and others have begun screening select OCD cohorts, including large families with multiple affected individuals, using whole exome and whole genome sequencing in order to identify other rare variants within glutamate genes.
Functional Role of SLC1A1
In addition to rare functional variants in SLC1A1, recent evidence suggests genomic regulation of isoform expression may be responsible for disruption of normal SLC1A1 function. Alternative splicing can generate a variety of mRNA and protein isoforms that have been shown to change the properties of protein function and, in turn, have meaningful physiological effects (Stamm et al., 2005). Recently, an internal promoter was identified upstream of exon 5 which was confirmed to drive the expression of a transcript consisting of exons 5 to 12 (isoform P2) (Porton et al., 2013). Along with P2, two additional isoforms were discovered, one missing exon 2 (ex2skip), the other exon 11 (ex11skip). In cell-lines, these isoforms were shown to reduce glutamate transport in transfection assays. Furthermore, the ex2skip and ex11skip partially co-localize and interact with the primary transcript. All three isoforms (P2, ex2skip, and ex11skip) are evolutionarily conserved between humans and mice, and were expressed in abundance relative to the primary transcript within the human striatum. Taken together, this strongly suggests that these SLC1A1 isoforms regulate glutamate transport in the striatum which may inform the interpretation of human genetic studies. Investigation into whether SNPs identified previously to be associated with OCD are influencing regulation of these isoforms is warranted.
SLC1A1/EAAT3 is part of a family of excitatory amino acid transporters (EAATs) which help remove glutamate from the neuronal synaptic cleft into neurons. Recently, inducible astrocyte-specific EAAT2 (GLT1) knock-out mice were shown to exhibit pathological repetitive behaviours (Aida et al., 2015). Treatment with memantine, an NMDA (N-methyl-D-aspartate) receptor antagonist, improved the repetitive behaviour in these mice. Prevention of glutamate reuptake in the synaptic cleft and, as a result, sustained glutamatergic neurotransmission would support the etiology proposed through the SAPAP3 studies described above. Studies have also shown that glutamatergic neurotransmission is coupled with neuronal glucose metabolism (Sibson et al., 1998). SLC1A1 was recently reported to be expressed in neuronal and glial mitochondria and participate in glutamate-stimulated ATP production (Magi et al., 2012). Thus, aberrant expression or regulation of this gene may influence neuronal metabolism and may explain the biological mechanism behind hyper-metabolism in the ACC (Anterior Cingulate Cortex) of OCD patients suggested by magnetic resonance spectroscopy (MRS) studies of OCD (Brennan, Rauch, Jensen, & Pope, 2013). Although further study is needed, recent evidence highlights a potential link between glutamate transport, neuronal metabolism, and OCD.
Glutamate Receptors: The GRIN and GRIK Families of NMDA receptors
While not as extensively studied as SLC1A1 or SAPAP3, a few variants within genes encoding glutamate receptors have also been associated with OCD. The NMDA receptors are responsible for the majority of excitatory synaptic transmission and plasticity in the central nervous system (Ozawa, Kamiya, & Tsuzuki, 1998). The glutamate receptor ionotropic NMDA receptor 2B gene (GRIN2B) is expressed at high levels in the fronto-parieto-temporal cortex, amygdala and the basal ganglia; all regions with reported abnormalities in neuroimaging studies of OCD (Milad & Rauch, 2012; Schito et al., 1997). Variants in GRIN2B have also been associated with other disorders affecting the brain such as ASD, ADHD, epilepsy and schizophrenia (Hu, Chen, Myers, Yuan, & Traynelis, 2016). In rats, expression of GRIN2B changes across development and this regulation is controlled by microRNAs (Corbel, Hernandez, Wu, & Kosik, 2015). This developmental change in expression may be of relevance for development of OCD symptoms in humans.
Results from studies of GRIN2B in OCD have been mixed. In a pilot study, Arnold et al. (2004) found a significant association between OCD and variants within the 3′UTR in GRIN2B, as well as an even stronger association with a haplotype block in the same region. Two more recent studies in two different ethnic populations (combined total of 431 OCD patients and 692 controls) revealed no association between variants in the 3′-UTR of GRIN2B and OCD in Han Chinese (Liu et al., 2012) or Spanish (Alonso et al., 2012) participants. In the latter study, group differences varied as a function of OCD sub-phenotypes. Specifically, one SNP in male patients and a four SNP haplotype in the whole sample were significantly associated with the presence of contamination obsessions and cleaning compulsions. Another more recent study investigated the role of rs1019385 in GRIN2B in a sample of Brazillian OCD patients and healthy controls. They found the T-allele to be significantly associated with ordering (P=0.03) and checking (P=0.03) symptoms (Kohlrausch et al., 2016). Although requiring replication, the association with specific OCD sub-phenotypes is intriguing, and future studies may benefit from a similar approach. OCD has a heterogeneous behavioral phenotype and different symptom clusters may have different genetic underpinnings (Miguel et al., 2005).
Glutamate receptor, ionotropic, kainate (GRIK) receptor genes (encoding the other type of ionotropic glutamate receptors) have also been reported to be associated with OCD, although specific allelic associations have not been consistent. A SNP in glutamate receptor, ionotropic, Kainate Receptor 2 (GRIK2) gene that was previously associated with autism, was also under-transmitted in OCD trios (Delorme et al., 2004). Following-up, Sampaio et al. studied 47 OCD probands and their parents in a family-based association study (Sampaio et al., 2011). While they failed to replicate the Delorme et al. study, they found a different SNP and a two-marker haplotype to be significantly associated with OCD. These studies are limited by their small sample sizes and so future studies are needed to clarify the association of these genes.
Imaging and Glutamate Genetics
Studying the association between genetic variants and neuroimaging has two advantages: 1) Imaging findings may represent intermediate phenotypes that are more homogeneous and closer to the action of genes compared with more complex behavioural phenotypes and therefore, at least in principle, provide added power for genetic studies; and 2) Imaging may shed light on mechanisms which mediate the influence of genetic variants on behaviour. Neuroimaging studies have identified abnormalities in brain structure, chemistry and function within cortico-striatal-thalamocortical (CSTC) circuits in patients with OCD. Structural magnetic resonance imaging (MRI) studies have revealed volumetric differences between cases and controls in a number of CSTC structures, particularly the orbitofrontal cortex (OFC), anterior cingulate cortex (ACC), thalamus and striatum. Proton magnetic resonance spectroscopy (1H-MRS) studies in psychotropic-naive children who suffer from OCD reported greater left, but not right, caudate Glx (glutamate+glutamine) concentrations (Rosenberg et al., 2000; Starck et al., 2008). In both pediatric and adult OCD patients lower ACC Glx has been observed regardless of medication status (Rosenberg et al., 2004; Yücel et al., 2008). These findings of increased caudate Glx and decreased ACC Glx have been supported in a systematic review of 14 MRS studies measuring glutamine (Gln) or glutamate (Glu) in OCD (Brennan et al., 2013).
Building upon findings of volumetric and Glx differences in the brains of patients with OCD compared with controls, and the association of glutamatergic genes with this disorder, our group and others have begun to investigate the potential role of glutamate genes in brain volume and glutamate concentration (K. Wu et al., 2012). In a structural magnetic resonance imaging (MRI) study of 20 psychotropic-naive pediatric OCD patients we measured volumes of brain regions selected a priori for their association with OCD (orbitofrontal cortex [OFC], ACC, thalamus, caudate, putamen, globus pallidus and pituitary). A total of 519 SNPs from 9 glutamatergic candidate genes (SAPAP1, SAPAP2, SAPAP3, GRIN2B, SLC1A1, GRIK2, GRIK3, SLITRK1 and SLITRK5) were tested for association with volumes of these regions. While no SNP remained significantly associated with volumetric changes after correction for multiple comparisons, the strongest finding was between two SNPs in DLGAP2 and OFC white matter volume (K. Wu et al., 2012).
In a study of Glx concentration (measured using 1H-MRS) in children with OCD, Arnold et al. (2009b) found a significant association between a GRIN2B SNP and Glx concentration in the ACC (Arnold et al., 2009). The variant associated with higher-risk for OCD in our earlier study (Arnold et al., 2004) was found more commonly in OCD patients correlating with the higher-risk phenotype of decreased ACC glutamatergic concentration (Arnold et al., 2009). These findings were consistent with the previously reported 1H-MRS findings of decreased Glx concentration in the ACC of OCD patients. Larger sample sizes and independent replication are needed to confirm findings from these imaging genetic studies. To date, all imaging genetic studies in OCD have adopted a candidate gene approach. However, other more comprehensive imaging genetics approaches such as a GWAS may also be fruitful in identifying other SNPs or genes associated with OCD. The reader is referred to “Imaging genetics-days of future past” for a more comprehensive review of other imaging-genetics approaches (Bigos & Weinberger, 2010). Currently, a consortium of investigators (including our group) is planning such a study as part of a larger cross-disorder initiative known as Enhancing NeuroImaging Genetics through Meta-Analysis (ENIGMA, http://enigma.ini.usc.edu/ongoing/enigma-ocd-working-group/).
Looking Forward
In this review, we have explored recent findings from human association studies and animal models in genes involved in excitatory synapse formation (SAPAP family), glutamate transport (SLC1A1), and glutamate receptors (GRIN and GRIK families). We believe that there is increasing evidence strengthening the case for a role for glutamate in OCD. Furthermore, we hypothesize that sustained glutamatergic neurotransmission in key parts of the brain may contribute the etiology of OCD. Future studies forthcoming from large consortia have larger sample sizes and better statistical power which will be helpful for additional gene discovery in OCD. Behaviorally, OCD is a very heterogeneous disorder which may reflect underlying genetic heterogeneity. Identification of intermediate phenotypes could be helpful in reducing this heterogeneity. Studying a more homogenous subset of OC traits across OCD and related disorders (e.g. TTM) may also help improve the significance and specificity of associations. Pathway analysis techniques help reduce complexity in genetic analysis and increase explanatory power (Khatri, Sirota, & Butte, 2012). This type of approach will allow us to interrogate a broader array of glutamate genes as a “system” thus assessing their collective impact on the disorder. For example, the Psychiatric Genomics Consortium recently used pathway analysis to find strong association between histone methylation processes and three adult psychiatric disorders (Network and Pathway Analysis Subgroup of Psychiatric Genomics Consortium, 2015). Associated pathways can then be further studied in animal models and in imaging genetic studies in humans. Glutamatergic agents such as riluzole, and memantine are already being assessed in OCD and are showing some promise (Grados, Atkins, Kovacikova, & McVicar, 2015). Elucidation of the role of glutamate genes in OCD may inform future treatment studies, both in the development of novel compounds as well as the stratification of patients for clinical trials based on genotype and phenotype. These studies may provide further support for the use of glutamate agents as treatments for OCD.
Acknowledgement / Conflicts of Interest
Dr. Arnold receives funding for his work from the Alberta Innovates Health Solutions (AIHS) Translational Health Chair in Child and Youth Mental Health.
References
- Aida T, Yoshida J, Nomura M, Tanimura A, Iino Y, Soma M, … Tanaka K. Astroglial glutamate transporter deficiency increases synaptic excitability and leads to pathological repetitive behaviors in mice. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology. 2015;40(7):1569–1579. doi: 10.1038/npp.2015.26. http://doi.org/10.1038/npp.2015.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso P, Gratacòs M, Segalàs C, Escaramís G, Real E, Bayés M, … Menchón JM. Association between the NMDA glutamate receptor GRIN2B gene and obsessive-compulsive disorder. Journal of Psychiatry & Neuroscience: JPN. 2012;37(4):273–281. doi: 10.1503/jpn.110109. http://doi.org/10.1503/jpn.110109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold PD, Macmaster FP, Richter MA, Hanna GL, Sicard T, Burroughs E, … Rosenberg DR. Glutamate receptor gene (GRIN2B) associated with reduced anterior cingulate glutamatergic concentration in pediatric obsessive-compulsive disorder. Psychiatry Research. 2009;172(2):136–139. doi: 10.1016/j.pscychresns.2009.02.005. http://doi.org/10.1016/j.pscychresns.2009.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold PD, Rosenberg DR, Mundo E, Tharmalingam S, Kennedy JL, Richter MA. Association of a glutamate (NMDA) subunit receptor gene (GRIN2B) with obsessive-compulsive disorder: A preliminary study. Psychopharmacology. 2004;174(4):530–538. doi: 10.1007/s00213-004-1847-1. http://doi.org/10.1007/s00213-004-1847-1. [DOI] [PubMed] [Google Scholar]
- Arnold PD, Sicard T, Burroughs E, Richter MA, Kennedy JL. Glutamate transporter gene SLC1A1 associated with obsessive-compulsive disorder. Archives of General Psychiatry. 2006;63(7):769–776. doi: 10.1001/archpsyc.63.7.769. http://doi.org/10.1001/archpsyc.63.7.769. [DOI] [PubMed] [Google Scholar]
- Association AP. The Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition.: DSM 5. American Psychiatric Association - Google Books; 2013. [Google Scholar]
- Bailey CG, Ryan RM, Thoeng AD, Ng C, King K, Vanslambrouck JM, … Rasko JE. Loss-of-function mutations in the glutamate transporter SLC1A1 cause human dicarboxylic aminoaciduria. The Journal of Clinical Investigation. 2011;121(1):446–453. doi: 10.1172/JCI44474. http://doi.org/10.1172/JCI44474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bienvenu OJ, Wang Y, Shugart YY, Welch JM, Grados MA, Fyer AJ, … Nestadt G. Sapap3 and pathological grooming in humans: Results from the OCD collaborative genetics study. American Journal of Medical Genetics Part B, Neuropsychiatric Genetics: The Official Publication of the International Society of Psychiatric Genetics. 2009;150B(5):710–720. doi: 10.1002/ajmg.b.30897. http://doi.org/10.1002/ajmg.b.30897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigos KL, Weinberger DR. Imaging genetics-days of future past. NeuroImage. 2010;53(3):804–809. doi: 10.1016/j.neuroimage.2010.01.035. http://doi.org/10.1016/j.neuroimage.2010.01.035. [DOI] [PubMed] [Google Scholar]
- Boardman L, van der Merwe L, Lochner C, Kinnear CJ, Seedat S, Stein DJ, … Hemmings SMJ. Investigating SAPAP3 variants in the etiology of obsessive-compulsive disorder and trichotillomania in the South African white population. Comprehensive Psychiatry. 2011;52(2):181–187. doi: 10.1016/j.comppsych.2010.05.007. http://doi.org/10.1016/j.comppsych.2010.05.007. [DOI] [PubMed] [Google Scholar]
- Brennan BP, Rauch SL, Jensen JE, Pope HG. A critical review of magnetic resonance spectroscopy studies of obsessive-compulsive disorder. Biological Psychiatry. 2013;73(1):24–31. doi: 10.1016/j.biopsych.2012.06.023. http://doi.org/10.1016/j.biopsych.2012.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burguière E, Monteiro P, Feng G, Graybiel AM. Optogenetic stimulation of lateral orbitofronto-striatal pathway suppresses compulsive behaviors. Science (New York, NY) 2013;340(6137):1243–1246. doi: 10.1126/science.1232380. http://doi.org/10.1126/science.1232380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M, Wan Y, Ade K, Ting J, Feng G, Calakos N. Sapap3 deletion anomalously activates short-term endocannabinoid-mediated synaptic plasticity. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2011;31(26):9563–9573. doi: 10.1523/JNEUROSCI.1701-11.2011. http://doi.org/10.1523/JNEUROSCI.1701-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbel C, Hernandez I, Wu B, Kosik KS. Developmental attenuation of N-methyl-D-aspartate receptor subunit expression by microRNAs. Neural Development. 2015;10(1):20. doi: 10.1186/s13064-015-0047-5. http://doi.org/10.1186/s13064-015-0047-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crane J, Fagerness J, Osiecki L, Gunnell B, Stewart SE, Pauls DL … Tourette Syndrome International Consortium for Genetics (TSAICG) Family-based genetic association study of DLGAP3 in Tourette Syndrome. American Journal of Medical Genetics Part B, Neuropsychiatric Genetics: The Official Publication of the International Society of Psychiatric Genetics. 2011;156B(1):108–114. doi: 10.1002/ajmg.b.31134. http://doi.org/10.1002/ajmg.b.31134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delorme R, Krebs M-O, Chabane N, Roy I, Millet B, Mouren-Simeoni MC, … Leboyer M. Frequency and transmission of glutamate receptors GRIK2 and GRIK3 polymorphisms in patients with obsessive compulsive disorder. Neuroreport. 2004;15(4):699–702. doi: 10.1097/00001756-200403220-00025. [DOI] [PubMed] [Google Scholar]
- Dickel DE, Veenstra-VanderWeele J, Cox NJ, Wu X, Fischer DJ, Van Etten-Lee M, … Hanna GL. Association testing of the positional and functional candidate gene SLC1A1/EAAC1 in early-onset obsessive-compulsive disorder. Archives of General Psychiatry. 2006;63(7):778–785. doi: 10.1001/archpsyc.63.7.778. http://doi.org/10.1001/archpsyc.63.7.778. [DOI] [PubMed] [Google Scholar]
- Grados MA, Atkins EB, Kovacikova GI, McVicar E. A selective review of glutamate pharmacological therapy in obsessive-compulsive and related disorders. Psychology Research and Behavior Management. 2015;8:115–131. doi: 10.2147/PRBM.S58601. http://doi.org/10.2147/PRBM.S58601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanna GL, Himle JA, Curtis GC, Gillespie BW. A family study of obsessive-compulsive disorder with pediatric probands. American Journal of Medical Genetics Part B, Neuropsychiatric Genetics: The Official Publication of the International Society of Psychiatric Genetics. 2005;134B(1):13–19. doi: 10.1002/ajmg.b.30138. http://doi.org/10.1002/ajmg.b.30138. [DOI] [PubMed] [Google Scholar]
- Hanna GL, Veenstra-VanderWeele J, Cox NJ, Boehnke M, Himle JA, Curtis GC, … Cook EH., Jr Genome-wide linkage analysis of families with obsessive-compulsive disorder ascertained through pediatric probands. American Journal of Medical Genetics. 2002;114(5):541–552. doi: 10.1002/ajmg.10519. http://doi.org/10.1002/ajmg.10519. [DOI] [PubMed] [Google Scholar]
- Harrison BJ, Soriano-Mas C, Pujol J, Ortiz H, López-Solà M, Hernández-Ribas R, … Cardoner N. Altered corticostriatal functional connectivity in obsessive-compulsive disorder. Archives of General Psychiatry. 2009;66(11):1189–1200. doi: 10.1001/archgenpsychiatry.2009.152. http://doi.org/10.1001/archgenpsychiatry.2009.152. [DOI] [PubMed] [Google Scholar]
- Hu C, Chen W, Myers SJ, Yuan H, Traynelis SF. Human GRIN2B variants in neurodevelopmental disorders. Journal of Pharmacological Sciences. 2016;132(2):115–121. doi: 10.1016/j.jphs.2016.10.002. http://doi.org/10.1016/j.jphs.2016.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Archives of General Psychiatry. 2005;62(6):593–602. doi: 10.1001/archpsyc.62.6.593. http://doi.org/10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- Khatri P, Sirota M, Butte AJ. Ten years of pathway analysis: Current approaches and outstanding challenges. PLoS Computational Biology. 2012;8(2):e1002375. doi: 10.1371/journal.pcbi.1002375. http://doi.org/10.1371/journal.pcbi.1002375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohlrausch FB, Giori IG, Melo-Felippe FB, Vieira-Fonseca T, Velarde LGC, de Salles Andrade JB, Fontenelle LF. Association of GRIN2B gene polymorphism and Obsessive Compulsive disorder and symptom dimensions: A pilot study. Psychiatry Research. 2016;243:152–155. doi: 10.1016/j.psychres.2016.06.027. http://doi.org/10.1016/j.psychres.2016.06.027. [DOI] [PubMed] [Google Scholar]
- Kwon S-K, Woo J, Kim S-Y, Kim H, Kim E. Transsynaptic adhesions between netrin-G ligand-3 (NGL-3) and receptor tyrosine phosphatases LAR, protein-tyrosine phosphatase delta (PTPdelta), and PTPsigma via specific domains regulate excitatory synapse formation. The Journal of Biological Chemistry. 2010;285(18):13966–13978. doi: 10.1074/jbc.M109.061127. http://doi.org/10.1074/jbc.M109.061127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J-M, Lu C-L, Cheng M-C, Luu S-U, Hsu S-H, Chen C-H. Exonic resequencing of the DLGAP3 gene as a candidate gene for schizophrenia. Psychiatry Research. 2013;208(1):84–87. doi: 10.1016/j.psychres.2012.12.015. http://doi.org/10.1016/j.psychres.2012.12.015. [DOI] [PubMed] [Google Scholar]
- Liu S, Yin Y, Liu Y, Sun Y, Zhang X, Ma X. Lack of an association between obsessive-compulsive disorder and polymorphisms in the 3′ untranslated region of GRIN2B in a Chinese Han population. Psychiatry Research. 2012;196(1):142–144. doi: 10.1016/j.psychres.2011.09.003. http://doi.org/10.1016/j.psychres.2011.09.003. [DOI] [PubMed] [Google Scholar]
- Magi S, Lariccia V, Castaldo P, Arcangeli S, Nasti AA, Giordano A, Amoroso S. Physical and functional interaction of NCX1 and EAAC1 transporters leading to glutamate-enhanced ATP production in brain mitochondria. PloS One. 2012;7(3):e34015. doi: 10.1371/journal.pone.0034015. http://doi.org/10.1371/journal.pone.0034015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattheisen M, Samuels JF, Wang Y, Greenberg BD, Fyer AJ, McCracken JT, … Nestadt G. Genome-wide association study in obsessive-compulsive disorder: Results from the OCGAS. Molecular Psychiatry. 2014 doi: 10.1038/mp.2014.43. http://doi.org/10.1038/mp.2014.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miguel EC, Leckman JF, Rauch S, do Rosario-Campos MC, Hounie AG, Mercadante MT, … Pauls DL. Obsessive-compulsive disorder phenotypes: Implications for genetic studies. Molecular Psychiatry. 2005;10(3):258–275. doi: 10.1038/sj.mp.4001617. http://doi.org/10.1038/sj.mp.4001617. [DOI] [PubMed] [Google Scholar]
- Milad MR, Rauch SL. Obsessive-compulsive disorder: Beyond segregated cortico-striatal pathways. Trends in Cognitive Sciences. 2012;16(1):43–51. doi: 10.1016/j.tics.2011.11.003. http://doi.org/10.1016/j.tics.2011.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monzani B, Rijsdijk F, Harris J, Mataix-Cols D. The structure of genetic and environmental risk factors for dimensional representations of DSM-5 obsessive-compulsive spectrum disorders. JAMA Psychiatry. 2014;71(2):182–189. doi: 10.1001/jamapsychiatry.2013.3524. http://doi.org/10.1001/jamapsychiatry.2013.3524. [DOI] [PubMed] [Google Scholar]
- Murray CJ, Lopez AD. The incremental effect of age-weighting on YLLs, YLDs, and DALYs: A response. Bulletin of the World Health Organization. 1996;74(4):445–446. [PMC free article] [PubMed] [Google Scholar]
- Network and Pathway Analysis Subgroup of Psychiatric Genomics Consortium. Psychiatric genome-wide association study analyses implicate neuronal, immune and histone pathways. Nature Neuroscience. 2015;18(2):199–209. doi: 10.1038/nn.3922. http://doi.org/10.1038/nn.3922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozawa S, Kamiya H, Tsuzuki K. Glutamate receptors in the mammalian central nervous system. Progress in Neurobiology. 1998;54(5):581–618. doi: 10.1016/s0301-0082(97)00085-3. [DOI] [PubMed] [Google Scholar]
- Pittenger C, Bloch MH, Williams K. Glutamate abnormalities in obsessive compulsive disorder: Neurobiology, pathophysiology, and treatment. Pharmacology & Therapeutics. 2011;132(3):314–332. doi: 10.1016/j.pharmthera.2011.09.006. http://doi.org/10.1016/j.pharmthera.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porton B, Greenberg BD, Askland K, Serra LM, Gesmonde J, Rudnick G, … Kao HT. Isoforms of the neuronal glutamate transporter gene, SLC1A1/EAAC1, negatively modulate glutamate uptake: Relevance to obsessive-compulsive disorder. Translational Psychiatry. 2013;3:e259. doi: 10.1038/tp.2013.35. http://doi.org/10.1038/tp.2013.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg DR, Keshavan MS. A.E. Bennett Research Award. Toward a neurodevelopmental model of obsessive-compulsive disorder. Biological Psychiatry. 1998;43(9):623–640. doi: 10.1016/s0006-3223(97)00443-5. [DOI] [PubMed] [Google Scholar]
- Rosenberg DR, MacMaster FP, Keshavan MS, Fitzgerald KD, Stewart CM, Moore GJ. Decrease in caudate glutamatergic concentrations in pediatric obsessive-compulsive disorder patients taking paroxetine. Journal of the American Academy of Child and Adolescent Psychiatry. 2000;39(9):1096–1103. doi: 10.1097/00004583-200009000-00008. http://doi.org/10.1097/00004583-200009000-00008. [DOI] [PubMed] [Google Scholar]
- Rosenberg DR, Mirza Y, Russell A, Tang J, Smith JM, Banerjee SP, … Moore GJ. Reduced anterior cingulate glutamatergic concentrations in childhood OCD and major depression versus healthy controls. Journal of the American Academy of Child and Adolescent Psychiatry. 2004;43(9):1146–1153. doi: 10.1097/01.chi.0000132812.44664.2d. http://doi.org/10.1097/01.chi.0000132812.44664.2d. [DOI] [PubMed] [Google Scholar]
- Sampaio AS, Fagerness J, Crane J, Leboyer M, Delorme R, Pauls DL, Stewart SE. Association between polymorphisms in GRIK2 gene and obsessive-compulsive disorder: A family-based study. CNS Neuroscience & Therapeutics. 2011;17(3):141–147. doi: 10.1111/j.1755-5949.2009.00130.x. http://doi.org/10.1111/j.1755-5949.2009.00130.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuels J, Wang Y, Riddle MA, Greenberg BD, Fyer AJ, McCracken JT, … Nestadt G. Comprehensive family-based association study of the glutamate transporter gene SLC1A1 in obsessive-compulsive disorder. American Journal of Medical Genetics Part B, Neuropsychiatric Genetics: The Official Publication of the International Society of Psychiatric Genetics. 2011;156B(4):472–477. doi: 10.1002/ajmg.b.31184. http://doi.org/10.1002/ajmg.b.31184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schirmbeck F, Zink M. Comorbid obsessive-compulsive symptoms in schizophrenia: Contributions of pharmacological and genetic factors. Frontiers in Pharmacology. 2013;4:99. doi: 10.3389/fphar.2013.00099. http://doi.org/10.3389/fphar.2013.00099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schito AM, Pizzuti A, Di Maria E, Schenone A, Ratti A, Defferrari R, … Mandich P. mRNA distribution in adult human brain of GRIN2B, a N-methyl-D-aspartate (NMDA) receptor subunit. Neuroscience Letters. 1997;239(1):49–53. doi: 10.1016/s0304-3940(97)00853-7. [DOI] [PubMed] [Google Scholar]
- Shugart YY, Wang Y, Samuels JF, Grados MA, Greenberg BD, Knowles JA, … Nestadt G. A family-based association study of the glutamate transporter gene SLC1A1 in obsessive-compulsive disorder in 378 families. American Journal of Medical Genetics Part B, Neuropsychiatric Genetics: The Official Publication of the International Society of Psychiatric Genetics. 2009;150B(6):886–892. doi: 10.1002/ajmg.b.30914. http://doi.org/10.1002/ajmg.b.30914. [DOI] [PubMed] [Google Scholar]
- Sibson NR, Dhankhar A, Mason GF, Rothman DL, Behar KL, Shulman RG. Stoichiometric coupling of brain glucose metabolism and glutamatergic neuronal activity. Proceedings of the National Academy of Sciences of the United States of America. 1998;95(1):316–321. doi: 10.1073/pnas.95.1.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamm S, Ben-Ari S, Rafalska I, Tang Y, Zhang Z, Toiber D, … Soreq H. Function of alternative splicing. Gene. 2005;344:1–20. doi: 10.1016/j.gene.2004.10.022. http://doi.org/10.1016/j.gene.2004.10.022. [DOI] [PubMed] [Google Scholar]
- Starck G, Ljungberg M, Nilsson M, Jönsson L, Lundberg S, Ivarsson T, … Carlsson ML. A 1H magnetic resonance spectroscopy study in adults with obsessive compulsive disorder: Relationship between metabolite concentrations and symptom severity. Journal of Neural Transmission (Vienna, Austria: 1996) 2008;115(7):1051–1062. doi: 10.1007/s00702-008-0045-4. http://doi.org/10.1007/s00702-008-0045-4. [DOI] [PubMed] [Google Scholar]
- Stewart SE, Fagerness JA, Platko J, Smoller JW, Scharf JM, Illmann C, … Pauls DL. Association of the SLC1A1 glutamate transporter gene and obsessive-compulsive disorder. American Journal of Medical Genetics Part B, Neuropsychiatric Genetics: The Official Publication of the International Society of Psychiatric Genetics. 2007;144B(8):1027–1033. doi: 10.1002/ajmg.b.30533. http://doi.org/10.1002/ajmg.b.30533. [DOI] [PubMed] [Google Scholar]
- Stewart SE, Mayerfeld C, Arnold PD, Crane JR, O’Dushlaine C, Fagerness JA, … Mathews CA. Meta-analysis of association between obsessive-compulsive disorder and the 3′ region of neuronal glutamate transporter gene SLC1A1. American Journal of Medical Genetics Part B, Neuropsychiatric Genetics: The Official Publication of the International Society of Psychiatric Genetics. 2013 doi: 10.1002/ajmg.b.32137. http://doi.org/10.1002/ajmg.b.32137. [DOI] [PubMed] [Google Scholar]
- Valleni-Basile LA, Garrison CZ, Jackson KL, Waller JL, McKeown RE, Addy CL, Cuffe SP. Frequency of obsessive-compulsive disorder in a community sample of young adolescents. Journal of the American Academy of Child and Adolescent Psychiatry. 1994;33(6):782–791. doi: 10.1097/00004583-199407000-00002. http://doi.org/10.1097/00004583-199407000-00002. [DOI] [PubMed] [Google Scholar]
- van Grootheest DS, Cath DC, Beekman AT, Boomsma DI. Twin studies on obsessive-compulsive disorder: A review. Twin Research and Human Genetics: The Official Journal of the International Society for Twin Studies. 2005;8(5):450–458. doi: 10.1375/183242705774310060. http://doi.org/10.1375/183242705774310060. [DOI] [PubMed] [Google Scholar]
- Veenstra-VanderWeele J, Xu T, Ruggiero AM, Anderson LR, Jones ST, Himle JA, … Arnold PD. Functional studies and rare variant screening of SLC1A1/EAAC1 in males with obsessive-compulsive disorder. Psychiatric Genetics. 2012;22(5):256–260. doi: 10.1097/YPG.0b013e328353fb63. http://doi.org/10.1097/YPG.0b013e328353fb63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan Y, Feng G, Calakos N. Sapap3 deletion causes mGluR5-dependent silencing of AMPAR synapses. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2011;31(46):16685–16691. doi: 10.1523/JNEUROSCI.2533-11.2011. http://doi.org/10.1523/JNEUROSCI.2533-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Adamczyk A, Shugart YY, Samuels JF, Grados MA, Greenberg BD, … Nestadt G. A screen of SLC1A1 for OCD-related alleles. American Journal of Medical Genetics Part B, Neuropsychiatric Genetics: The Official Publication of the International Society of Psychiatric Genetics. 2010;153B(2):675–679. doi: 10.1002/ajmg.b.31001. http://doi.org/10.1002/ajmg.b.31001. [DOI] [PubMed] [Google Scholar]
- Welch JM, Lu J, Rodriguiz RM, Trotta NC, Peca J, Ding J-D, … Feng G. Cortico-striatal synaptic defects and OCD-like behaviours in Sapap3-mutant mice. Nature. 2007;448(7156):894–900. doi: 10.1038/nature06104. http://doi.org/10.1038/nature06104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendland JR, Moya PR, Timpano KR, Anavitarte AP, Kruse MR, Wheaton MG, … Murphy DL. A haplotype containing quantitative trait loci for SLC1A1 gene expression and its association with obsessive-compulsive disorder. Archives of General Psychiatry. 2009;66(4):408–416. doi: 10.1001/archgenpsychiatry.2009.6. http://doi.org/10.1001/archgenpsychiatry.2009.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willour VL, Yao Shugart Y, Samuels J, Grados M, Cullen B, Bienvenu OJ, … Nestadt G. Replication study supports evidence for linkage to 9p24 in obsessive-compulsive disorder. American Journal of Human Genetics. 2004;75(3):508–513. doi: 10.1086/423899. http://doi.org/10.1086/423899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H, Wang X, Xiao Z, Yu S, Zhu L, Wang D, … Fralick D. Association between SLC1A1 gene and early-onset OCD in the Han Chinese population: A case-control study. Journal of Molecular Neuroscience: MN. 2013;50(2):353–359. doi: 10.1007/s12031-013-9995-6. http://doi.org/10.1007/s12031-013-9995-6. [DOI] [PubMed] [Google Scholar]
- Wu K, Hanna GL, Easter P, Kennedy JL, Rosenberg DR, Arnold PD. Glutamate system genes and brain volume alterations in pediatric obsessive-compulsive disorder: A preliminary study. Psychiatry Research. 2012 doi: 10.1016/j.pscychresns.2012.07.003. http://doi.org/10.1016/j.pscychresns.2012.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yücel M, Wood SJ, Wellard RM, Harrison BJ, Fornito A, Pujol J, … Pantelis C. Anterior cingulate glutamate-glutamine levels predict symptom severity in women with obsessive-compulsive disorder. The Australian and New Zealand Journal of Psychiatry. 2008;42(6):467–477. doi: 10.1080/00048670802050546. http://doi.org/10.1080/00048670802050546. [DOI] [PubMed] [Google Scholar]
- Zarate CA, Manji HK. The role of AMPA receptor modulation in the treatment of neuropsychiatric diseases. Experimental Neurology. 2008;211(1):7–10. doi: 10.1016/j.expneurol.2008.01.011. http://doi.org/10.1016/j.expneurol.2008.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Züchner S, Wendland JR, Ashley-Koch AE, Collins AL, Tran-Viet KN, Quinn K, … Murphy DL. Multiple rare SAPAP3 missense variants in trichotillomania and OCD. Molecular Psychiatry. 2009;14(1):6–9. doi: 10.1038/mp.2008.83. http://doi.org/10.1038/mp.2008.83. [DOI] [PMC free article] [PubMed] [Google Scholar]