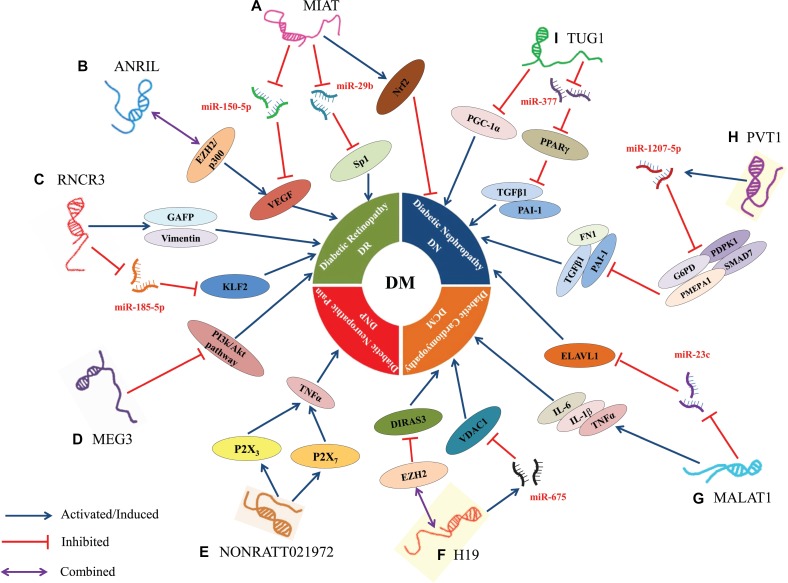
Figure 2. Dysregulation and functional roles of LncRNAs in DM.
(A) MIAT regulated microvascular dysfunction via a ceRNA regulatory network MIAT/miR-150-5p/VEGF in diabetic retinas and endothelial cells; MIAT promoted cell apoptosis by absorbing miR-29b and subsequently relieving its repressive effect on the targeting genes Sp1 in high glucose stimulated rat retinal Müller cells (rMC-1); MIAT suppressed high glucose-induced tubular damage by promoting the expression of Nrf2 in human renal tubular epithelial cell line (HK-2). (B) ANRIL regulated VEGF expression prompted the development of DN by directly binding the p300 and EZH2 complex. (C) RNCR3 promoted DM-induced retinal neurodegeneration because of retinal cell apoptosis and Müller glial cell proliferation via the increased expression of glial reactivity-related genes including GFAP and vimentin; RNCR3 regulated endothelial cell function through RNCR3/miR-185-5p/KLF2 regulatory network to promote DM-induced retinal microvascular abnormalities. (D) MEG3 inhibited retinal endothelial cell proliferation, migration, and tube formation by suppressing the activation of PI3k/Akt signalling in the retinas of STZ-induced diabetic mice. (E) NONRATT021972 could interact with P2X3 or P2X7 to induce the release of inflammatory factors (TNF-α), thereby resulting in the excitability of DRG neurons to increase the risk of DNP. (F) H19 inhibited autophagy in cardiomyocytes exposed to high glucose by recruiting EZH2 and subsequently suppressing DIRAS3 expression; H19, a host gene of miR-675, also suppressed high glucose-induced cardiomyocyte apoptosis by reducing the expression of miR-675 target gene VDAC1. (G) MALAT1 attenuated the diabetes-induced myocardial inflammation in myocardial tissue of diabetic rats by reducing the levels of inflammatory markers such as TNF-α, IL-1β and IL-6; MALAT1 facilitated renal tubular epithelial pyroptosis by modulated miR-23c targeting of ELAVL1 in STZ-induced diabetic rats. (H) PVT1, a host gene of miR-1207-5p, suppressed the expression of miR-1207-5p targeting genes (G6PD, PMEPA1, PDPK1, and SMAD7), thereby increasing the expression of ECM-related genes FN1, PAI-1 and TGF-β1 in mesangial cells. (I) TUG1 regulated the metabolism of DN by epigenetic targeting of expression of PGC-1α in podocytes; TUG1 suppressed ECM accumulation via TUG1/miR-377/PPARγ signalling, and was also involved in reciprocally suppressing miR-377 and increasing the expression of targeted gene PPARγ to decrease the ECM accumulation in mesangial cell. In this diagram, the symbol “ ”represents activated or induced; the symbol “
”represents activated or induced; the symbol “ ”represents inhibited; and the symbol “
”represents inhibited; and the symbol “ ” represents combined or sponged.
” represents combined or sponged.