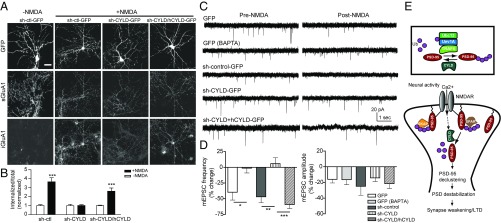
Fig. 9.
CYLD is required for cLTD. (A) NMDA (50 μM, 9 min)-triggered sGluA1 internalization in cultured rat hippocampal neurons infected with indicated plasmids. (Scale bar, 30 μm.) (B) Quantification of A, normalized to respective no-NMDA controls. n = 13–19 cells; ***P < 0.001; unpaired t tests. (C) NMDA-triggered depression of mEPSCs in cultured rat hippocampal neurons transfected with indicated plasmids. Intracellular BAPTA: 15 mM. (D) Quantification of mEPSC frequency and amplitude. n = 6–8 cells; ***P < 0.001, **P < 0.01, *P < 0.05; one-way ANOVA with post hoc Tukey’s test. (E) Model for roles of nonproteolytic K63 linkage in the synapse using PSD-95 as a proof of principle. (Upper) An E2/E3/DUB complex controls the balance of K63 ubiquitination/deubiquitination of PSD-95. (Lower) K63-polyUb plays an essential role in synaptic maintenance and orderly organization of PSD-95 and LTD. PSD-95 is constitutively conjugated by K63-polyUb chains (by Ubc13/Uev1A–TRAF6), which maintains and compartmentalizes the protein (perhaps combined with other posttranslational modifications) to specific subdomains within the PSD. Synaptic activity opens NMDARs, allowing Ca2+ influx, and recruits/activates CYLD (dashed arrow), which subsequently removes K63-polyUb from PSD-95. Deubiquitinated PSD-95 translocates away from PSD, destabilizing the PSD and weakening the synapse. Palmitoylated PSD-95 is targeted to the synapse membrane but may not be incorporated in a particular scaffolding/signaling complex that depends on PSD-95 K63 ubiquitination.