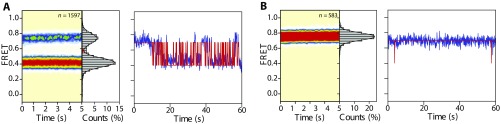
Fig. S1.
S13/tRNA-labeled POST complexes dynamically exchange between classical and hybrid states with peptidyl-tRNA bound within the P site. (A, Left) Population FRET histogram of S13/tRNA-labeled POST complexes bearing fMet-Phe-tRNAPhe programmed with a cognate (UUC) codon. (A, Right) Representative S13/tRNA FRET trace for the POST complex. (B, Left) Population FRET histogram of the POST complex shown in A following peptide release by incubation with puromycin (10 min, 25 °C, 2 mM puromycin). (B, Right) Representative S13/tRNA FRET trace for the puromycin-released POST complex. Comparing Fig.2 B and C, we observe that the stability of these hybrid-like peptidyl-tRNA configurations is sensitive to the nature of the nascent peptide. Data were acquired at a 40-ms time resolution. The number (n) of individual FRET trajectories in each histogram is indicated.